Translate this page into:
Increased tissue leptin hormone level and mast cell count in skin tags: A possible role of adipoimmune in the growth of benign skin growths
2 Department of Histology, Faculty of Medicine, Cairo University, Egypt
3 Department of Clinical Biochemistry, Faculty of Medicine, Cairo University, Egypt
Correspondence Address:
Rania M Abdel Hay
13th Abrag Othman,Kournish El Maadi, Cairo (11431)
Egypt
| How to cite this article: El Safoury O, Fawzi M, Abdel Hay RM, Hassan AS, El Maadawi Z, Rashed L. Increased tissue leptin hormone level and mast cell count in skin tags: A possible role of adipoimmune in the growth of benign skin growths. Indian J Dermatol Venereol Leprol 2010;76:538-542 |
Abstract
Background: Skin tags (ST) are common tumors. They mainly consist of loose fibrous tissue and occur on the neck and major flexures as small, soft, pedunculated protrusions. Decrease in endocrine, hormone level and other factors are thought to play a role in the evolution of ST. Leptin is an adipocyte-derived hormone that acts as a major regulatory hormone for food intake and energy homeostasis. Leptin deficiency or resistance can result in profound obesity and diabetes in humans. A role of mast cell in the pathogenesis of ST is well recognized. Aims: To investigate the role of leptin in the pathogenesis of ST and to clarify whether there is a correlation between mast cell count and leptin level in ST. Methods: Forty-five skin biopsies were taken from 15 patients with ST. From each patient, a biopsy of a large ST (length >4 mm), a small ST (length <2 mm) and a normal skin biopsy (as a control) were taken. The samples were processed for leptin level. Skin biopsies were stained with hematoxylin and eosin and toluidine blue-uranyl nitrate metachromatic method for mast cell count was used. Results: There was a significant increased level of leptin in the ST compared to the normal skin. It was highly significant in small ST than in big ST (P = 0.0001) and it was highly significant in small and big ST compared to controls, P = 0.0001 and P = 0.001, respectively. There was a significant increase in mast cell count in the ST, which did not correlate with the increased levels of leptin. Conclusion: This is the first report to demonstrate that tissue leptin may play a role in the pathogenesis of ST. The significant increase in the levels of leptin and mast cell count in ST may indicate a possible role of adipoimmune in the benign skin growths.Introduction
Skin tag (ST), also known as soft fibroma or acrochordon, a common benign condition, occurs mainly on the neck and major flexures as a small, soft, pedunculated protrusion. [1] Histopathologically, ST is a polypoid lesion with loose to dense fibrovascular core together with an overlying mildly acanthotic epidermis. ST often develops in areas of friction [2] and is associated with several conditions, including diabetes mellitus, [3] obesity, [4] acromegaly, [5] Crohn′s disease, [6] aging, [7] child abuse, [8] organ transplants, [9] colonic polyps, [10] pregnancy, [11] human papilloma virus, [12] increased mast cell count [13] and increased androgen and estrogen receptors in ST. [14] Leptin is a protein secreted by adipose tissue, which has an important role in metabolism and immunity. It regulates body weight and other functions that modulate hematopoiesis, angiogenesis and immune responses. [15] Additionally, leptin mediates proliferative and antiapoptotic activities in different cell types, including T cells, macrophages [16] and eosinophils. [17]
Leptin receptor is expressed primarily in the hypothalamus, but it is also expressed by peripheral blood mononuclear cells, vascular endothelial cells, smooth muscle cells, osteoblasts and fibroblasts. [18]
The aim of this study is to investigate the level of leptin hormone in ST in an attempt to elucidate a possible role of leptin in the pathogenesis of ST. Furthermore, this work explores the correlation between leptin levels and mast cell count in the ST.
Methods
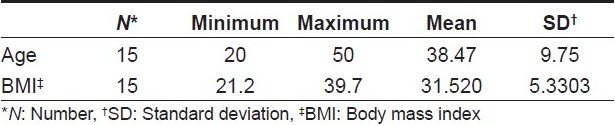
Fifteen nondiabetic participants seeking advice for their ST were enrolled in this study. This study was conducted in the Dermatology Outpatient Clinic, from August 2008 to December 2008, and was approved by the Dermatology Research Ethics Committee and Faculty of Medicine, Cairo University. All participants were asked to sign an informed consent prior to inclusion in the study. For determining related diseases, the participants were subjected to thorough history taking and clinical examinations like measurement of body mass index (BMI) [Table - 1] using the following equation: [19]
BMI = weight (kg)/height 2 (m 2 )
From each participant, three skin biopsies were taken as follow: a large ST (length >4 mm), a small ST (length <2 mm) and a normal skin biopsy (as a control). Skin biopsies were stained with hematoxylin and eosin and toluidine blue-uranyl nitrate metachromatic method for mast cell count was used.
Sample collection and preparation for leptin measurement
Skin biopsies were homogenized using a tissue homogenizer. After cell lysis, the homogenate was centrifuged at 10,000 Χ g for 20 min at 4C and the supernatant was stored at -80C until performance of the assay.
Measurement of leptin level in ST
The supernatant was examined for leptin using a quantitative sandwich enzyme-linked immunoassay, which was supplied by RayBiotech Inc., Norcross, GA, USA.
Mast cell count
The Toluidine blue-stained sections were examined under an Olympus microscope and 10 different fields of each section were counted manually by the same person [Figure - 1] and [Figure - 2].
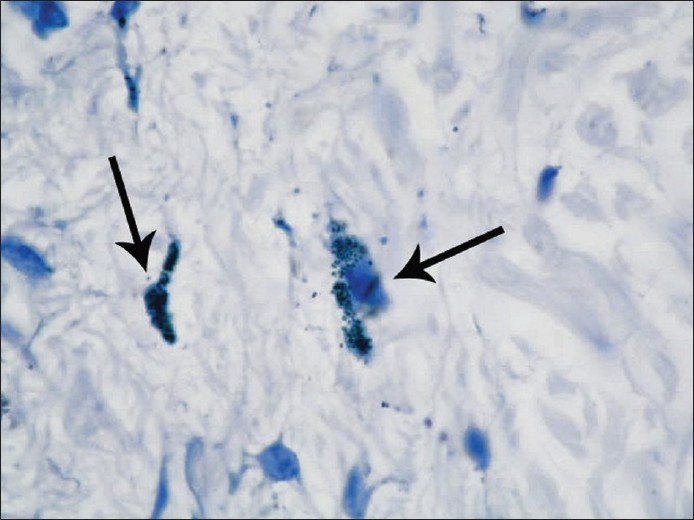 |
| Figure 1 :Mast cells stained metachromatically with Toluidine blue (black arrows) in the dermis of normal skin (Toluidine blue, x1,000) |
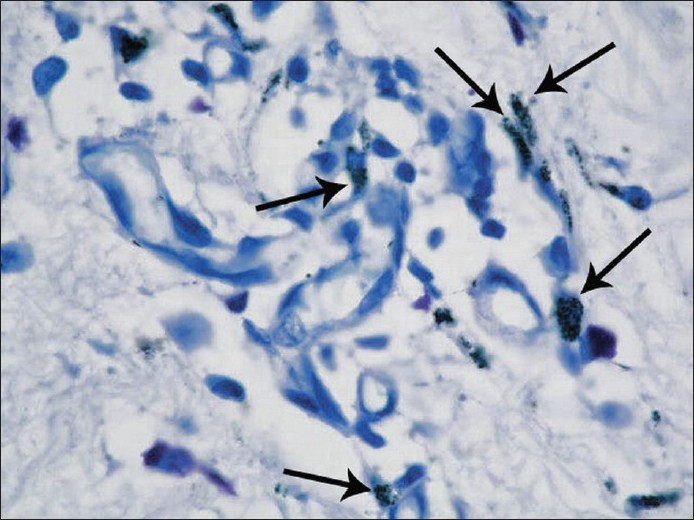 |
| Figure 2 :Mast cells stained metachromatically with Toludine blue (black arrows) in the dermis of a large skin tag close to the blood vessels (Toluidine blue, x1,000) |
Statistics
Data were statistically described in terms of range, mean standard deviation (SD), median, frequencies (number of cases) and relative frequencies (percentages) when appropriate. Comparison of leptin and mast cell count between ST and control skin was carried out using Freidman′s test with Wilcoxon-signed rank test for paired (matched) samples as post hoc multiple 2-group comparisons. Correlation between various variables was performed using the Spearman rank correlation equation for non normal variables. A probability value (P-value) <0.05 was considered statistically significant. All statistical calculations were carried out using computer programs Microsoft Excel version 7 (Microsoft Corporation, Cary, NY, USA) and SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows. Arcus QuickStat (Biomedical), Arcus statistical software, Research Solutions, Addison Wesley Longman Ltd., Toronto, ON, USA.
Results
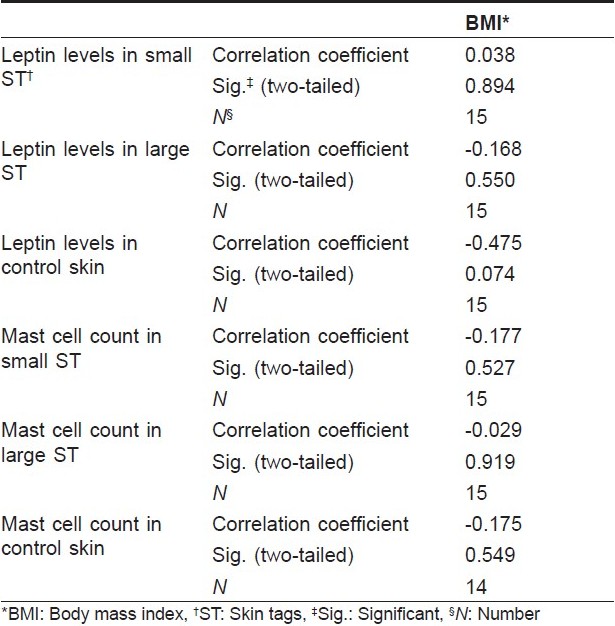
There was a significant increase of tissue leptin levels in small ST when compared to the control group (P = 0.0001) and in big ST when compared to the control group (P = 0.001) [Figure - 3]. Leptin levels were significantly higher in small ST when compared to big ST (P = 0.0001). The number of mast cell count/high power field (HPF) was significantly higher in ST when compared to the control (P = 0.0178) [Figure - 4]. The correlation of mast cell count with leptin level in ST and control was statistically nonsignificant [Table - 1]. Furthermore, the correlation of leptin level and mast cell count/HPF with BMI was not significant [Table - 2].
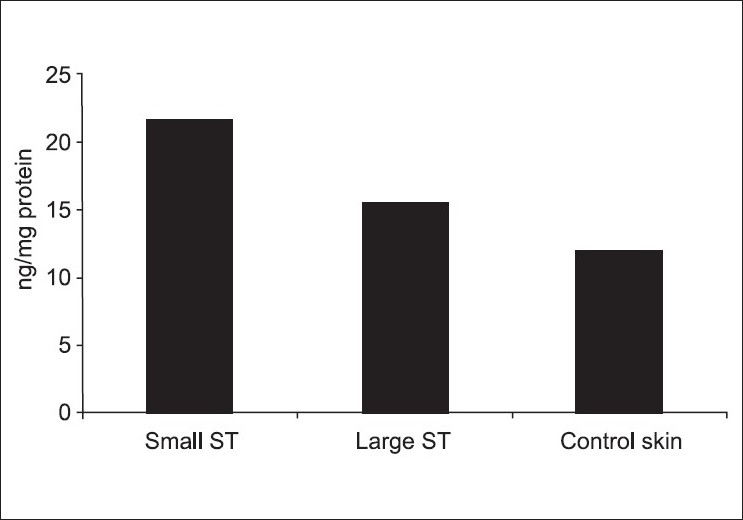 |
| Figure 3 :Difference in tissue leptin level (ng/mg protein) in small skin tag (ST), large ST and control skin. Values are described as mean ± standard deviation |
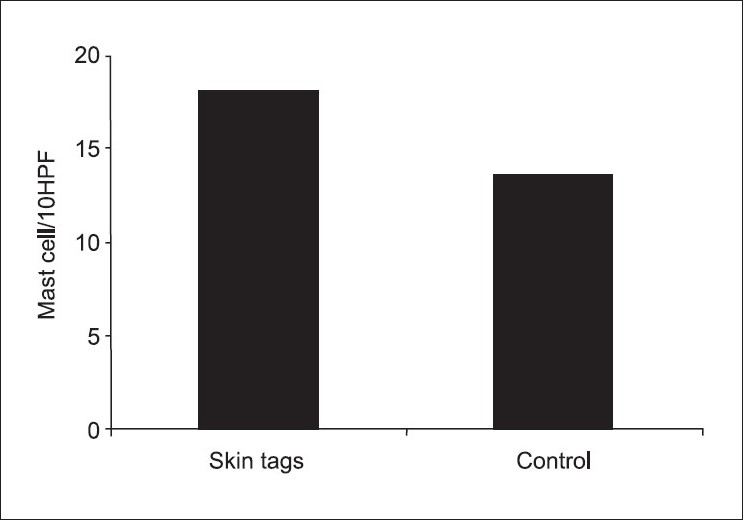 |
| Figure 4 :Significant difference in mast cell count/10 HPF in skin tags and control skin. Values are described as mean ± standard deviation |
Discussion
Leptin, a 16-kDa protein, is produced primarily by adipocytes and acts as a major regulator for food intake and energy homeostasis. Leptin deficiency or resistance can result in profound obesity, diabetes and infertility in humans. [15] Since leptin discovery, its biological functions has expanded from anti obesity to broad effects on reproduction, hematopoiesis, angiogenesis, blood pressure, bone mass, lymphoid organ homeostasis and T-lymphocyte system. [16],[17] Leptin is thought to link the nutritional status with neuroendocrine and immune functions, and its role in the modulation of immune response and inflammation has recently become increasingly evident. Leptin orchestrates complex biological effects through its class I cytokine receptor superfamily, expressed both centrally and peripherally. [20]
Skin tags are considered the most common fibrous lesions of the skin. However, they have received little attention in the dermatological literature and, to date, their exact etiology is not fully understood. A relation to obesity, diabetes mellitus, friction, acromegaly, organ transplant, human papilloma virus and other conditions has been reported. [13] However, how these factors may cause the development of ST is not yet clear. In this study, we attempted to measure the level of leptin hormone in tissue of ST to investigate its role in the pathogenesis of these benign lesions. Interestingly, there was a significant increase of leptin levels in ST compared to control skin. Furthermore, leptin hormone levels were found to be significantly higher in small ST compared to large ST. As the ST protrudes out, proliferation of the epidermis together with the core of the dermis occur. Previous studies have shown that leptin acts as an angiogenic factor in the vascular endothelium. [21] Fibroblasts and keratinocytes are also known to express leptin receptors, and the mitogenic effect of leptin on keratinocytes has been recently demonstrated during wound healing by in vivo studies. [22] Furthermore, the role of leptin in the proliferation of both normal and cancer cells has been previously reported. [23] It is therefore plausible that the increased levels of leptin in ST, as shown in this study, through its angiogenic effect and the induction of cellular proliferation, may play an important role in the development of ST. Accordingly, the higher level of tissue leptin found in small ST compared to big ST in our findings may correspond to the increased proliferation potential of early developing small ST compared to the more stable larger lesions. It is important to note that increased levels of leptin have also been reported with obesity, [20] diabetes mellitus [24] and pregnancy, [25] all of which have been linked with the development of ST. Taken together, these findings may indicate a novel role of leptin hormone in the evolution of ST. Plasma leptin level was reported to correlate strongly with BMI; [25] yet, in this study, we demonstrated that tissue leptin may not correlate with BMI. It was reported that plasma leptin level correlates with the number of adipose tissue mast cells in patients with metabolic syndrome. [26],[27] Furthermore, a possible role of mast cells in the pathogenesis of ST was previously demonstrated by investigators from our group. [13] In this work, mast cell count/HPF cell were significantly increased in ST when compared to normal controls. However, this increase did not significantly correlate with increased tissue leptin levels. Nevertheless, the possible stimulatory action of tissue leptin on mast cell growth requires further investigation, especially because mast cells were recently shown to express leptin receptors. [28]
To our knowledge, this the first study to investigate the role of leptin in ST and/or other benign skin growths. The present findings indicate a possible adipoimmune role in the pathogenesis of ST. Future studies are warranted to elucidate the exact role of leptin in the development of ST and other benign skin growths.
Acknowledgment
We are indebted to Prof. Magdy Ibrahim, MD, Professor of Statistical Unit, Faculty of Medicine, Cairo University, for his statistical analysis of this work.
| 1. |
Allegue F, Fachal C, Pιrez-Pιrez L. Friction Induced Skin Tags. Dermatol Online J 2008;14:18.
[Google Scholar]
|
| 2. |
Gupta S, Aggarwal R, Gupta S, Arora SK. Human papillomavirus and skin tags: Is there any association? Indian J Dermatol Venereol Leprol 2008;74:222-5.
[Google Scholar]
|
| 3. |
Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol 1987;67:175-7.
[Google Scholar]
|
| 4. |
Garcia-Hidalgo L, Orozco-Tropete R, Gonzalez-Barranco J, Villa AR, Dalman JJ, Ortiz-Pedroza G. Dermatoses in 156 obese adults. Obes Res 1999;7:299-302.
[Google Scholar]
|
| 5. |
Ben-Shlomo A, Melmed S. Skin manifestations in acromegaly. Clin Dermatol 2006;24:256-9.
[Google Scholar]
|
| 6. |
Singh B, McC Mortensen NJ, Jewell DP, George B. Perianal Crohn's disease. Br J Surg 2004;91:801-4.
[Google Scholar]
|
| 7. |
Turner ML. Skin changes after forty. Am Fam Physician 1984;29:173-81.
[Google Scholar]
|
| 8. |
Muram D. Anal and perianal abnormalities in prepubertal victims of sexual abuse. Am J Obstet Gynecol 1989;161:278-81.
[Google Scholar]
|
| 9. |
Euvrard S, Kanitakis J, Cochat P, Cambazard F, Claudy A. Skin diseases in children with organ transplants. J Am Acad Dermatol 2001;44:932-9.
[Google Scholar]
|
| 10. |
Leavitt J, Klein I, Kendricks F, Gavaler J, VanThiel DH. Skin tags: a cutaneous marker for colonic polyps. Ann Intern Med 1983;98:928-30.
[Google Scholar]
|
| 11. |
Errickson CV, Matus NR. Skin disorders of pregnancy. Am Fam Physician 1994;49:605-10.
[Google Scholar]
|
| 12. |
Dianzani C, Calvieri S, Pierangeli A, Imperi M, Bucci M, Degener AM. The detection of human papillomavirus DNA in skin tags. Br J Dermatol 1998;138:649-51.
[Google Scholar]
|
| 13. |
Zaher H, El Safoury OS, El Komy MM, Mahmoud SB, Abd El Hamid H. Study of mast cell count in skin tags. Indian J Dermatol 2007;52:184-7.
[Google Scholar]
|
| 14. |
El Safoury O, Rashid L, Ibrahim M. A study of androgen and estrogen receptors a, b in skin tags. Indian J Dermatol 2010;1:20-4.
[Google Scholar]
|
| 15. |
Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gσmez-Reino JJ, et al. Leptin from fat to inflammation: old questions and new insights. FEBS Lett 2005;579:295-301.
[Google Scholar]
|
| 16. |
Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, et al. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol 2002;128:21-6.
[Google Scholar]
|
| 17. |
Conus C, Bruno A, Simon HU. Leptin is an eosinophil survivor factor. J Allergy Clin Immunol 2005;116:1128-34.
[Google Scholar]
|
| 18. |
Glasow A, Kiess W, Anderegg U, Berthold A, Bottner A, Kratzsch J. Expression of leptin (Ob) and leptin receptor (Ob-R) in human fibroblasts: regulation of leptin secretion by insulin. J Clin Endocrinol Metab 2001;86:4472-9.
[Google Scholar]
|
| 19. |
Eknoyan G. Adolphe Quetelet (1796-1874) -- the average man and indices of obesity. Nephrol Dial Transplant 2008;23:47-51.
[Google Scholar]
|
| 20. |
Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm 2005;71:345-72.
[Google Scholar]
|
| 21. |
Sierra-Honigmann MR, Nath AK, Murakami C, Garcνa-Cardeρa G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science 1998;281:1683-6.
[Google Scholar]
|
| 22. |
Stallmeyer B, Kδmpfer H, Podda M, Kaufmann R, Pfeilschifter J, Frank S. A novel keratinocyte mitogen: regulation of leptin and its functional receptor in skin repair. J Invest Dermatol 2001;117:98-105.
[Google Scholar]
|
| 23. |
Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin-a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 2002;94:1704-11.
[Google Scholar]
|
| 24. |
Luna R, Garcia-Mayor RV, Lage M, Andrade MA, Barreiro J, Pombo M, et al. High serum leptin levels in children with type 1 diabetes mellitus: Contribution of age, BMI, pubertal development and metabolic status. Clin Endocrinol 1999;51:603-10.
[Google Scholar]
|
| 25. |
Tamαs P, Sulyok E, Szabσ I, Vizer M, Ertl T, Rascher W, et al. Changes of maternal serum leptin levels during pregnancy. Gynecol Obstet Invest 1998;46:169-71.
[Google Scholar]
|
| 26. |
Lee JH, Reed DR, Price RA. Leptin resistance is associated with extreme obesity and aggregates in families. Int J Obesity Related Metab Disord 2001;25:1471-3.
[Google Scholar]
|
| 27. |
Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes 2002;5:1005-15.
[Google Scholar]
|
| 28. |
Taildeman J, Pιrez-Novo CA, Rottiers I, Ferdinande L, Waeytens A, De Colvenaer V, et al. Human mast cells express leptin and leptin receptors. Histochem Cell Biol 2009;131:703-11.
[Google Scholar]
|
Fulltext Views
3,498
PDF downloads
2,386





