Translate this page into:
Interferons
Correspondence Address:
Sandeep Kaur
Skin OPD, 1st Floor, OPD Block, GGS Medical Hospital, Sadiq Road, Faridkot, Punjab - 151 203
India
| How to cite this article: Mahajan BB, Kaur S. Interferons. Indian J Dermatol Venereol Leprol 2015;81:51-55 |
INTRODUCTION
Interferons (IFNs) are glycoproteins belonging to the family of cytokines and have antiviral, antitumor, and immunomodulatory activities. Nagano and Kojima, Japanese virologists, noticed viral growth inhibition in an area previously inoculated with virus and named it as "viral inhibitory factor" but their work was not fully appreciated. The main credit for discovering interferons has gone to Isaacs and Lindenmann who also coined the term "interferon."
CLASSIFICATION
Interferons are classified based on receptor binding and structural and biochemical differences [Table - 1].
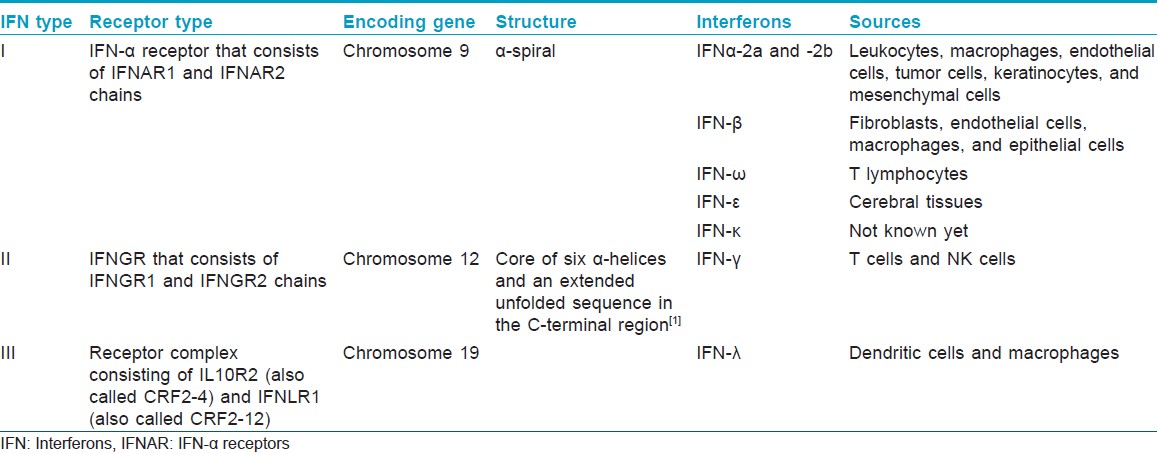
On the basis of receptor binding there are three types of interferons:
IFN type I: Binds to IFN-α receptor (IFNAR) that consists of IFNAR1 and IFNAR2 chains.
IFN type II: Binds to IFNGR that consists of IFNGR1 and IFNGR2 chains.
IFN type III: Signals through a receptor complex consisting of IL10R2 and IFNLR1.
Action Spectrum
Interferons are known to exhibit a myriad of activities which are depicted in [Figure - 1]. These include
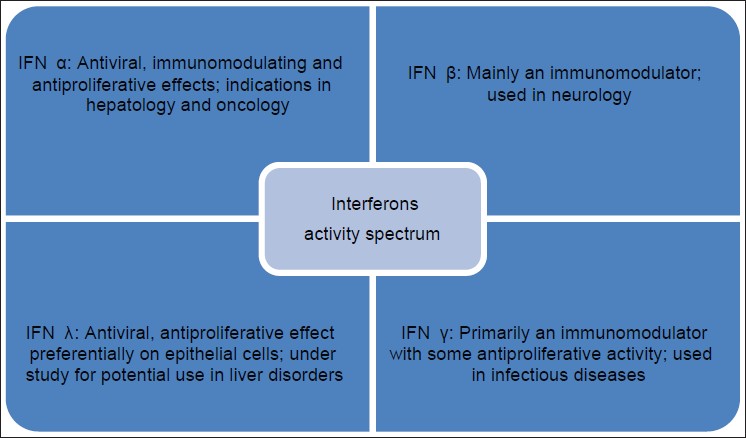 |
| Figure 1: Action spectrum of interferons, IFN α , β , γ have been used in dermatology |
Antiviral activity
Interferons have potent, but non-specific antiviral activity through inhibition of virus entry, viral protein synthesis and virus maturation and through induction of assembly defects.
Anti-tumor activity
This results from modulation of cell proliferation and differentiation, tumor antigen expression, and induction of class I and II antigen expression by tumor cells. Interferons have cytostatic effect causing arrest in G1 phase of cell cycle along with inhibition of certain proto-oncogenes and induction of apoptosis.
Immunomodulating activity
Interferons induce differentiation and activation of natural killer (NK) cells, monocytes, and macrophages (interferon-γ and -λ) and dendritic cells (interferon-α and -λ). They also enhance cytotoxic T-cell activity and increase the production of immunoglobulins (interferon-γ). Interferon-β and -γ inhibit the production of interleukin-10, while interferon-λ increases it. Interferon-γ inhibits the synthesis of interleukin-4, but increases the synthesis of tumor necrosis factor alpha (TNF-α) and interleukin-2.
Other activities
Interferons also exhibit anti-angiogenic activity through inhibition of vascular endothelial cells.
Interferon-λ has been shown to inhibit keratinocyte proliferation.
PHARMACOKINETICS
Various routes of administration of interferons include subcutaneous (SC), intramuscular (IM), intralesional, aerosols, topical, and possibly intravenous. Bioavailability is over 80% for IFN-α, but only 20-40% for IFN-β and 30-70% for IFN-γ. These are metabolized in the liver and eliminated by the kidneys.
Peak plasma concentrations are achieved in 1-12 h with plasma half-life of <12 h. Nowadays, interferons are conjugated with polyethylene glycol (pegylation). This process increases their molecular weight and delays renal clearance, thereby leading to a longer half-life.
Indications In Dermatology
[Table - 2] and [Table - 3] describe the indications of interferons in dermatology.
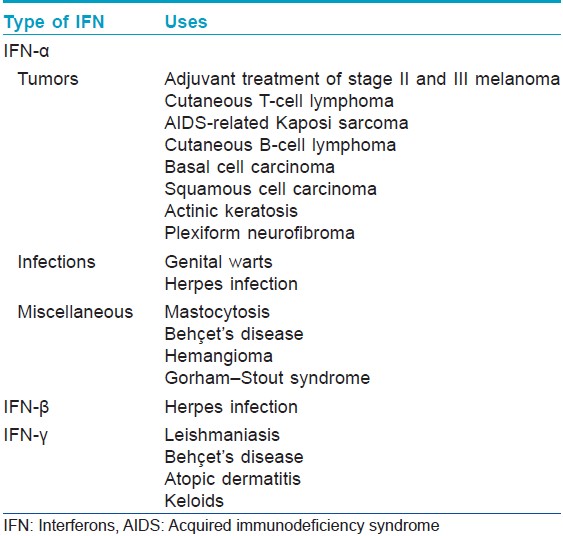
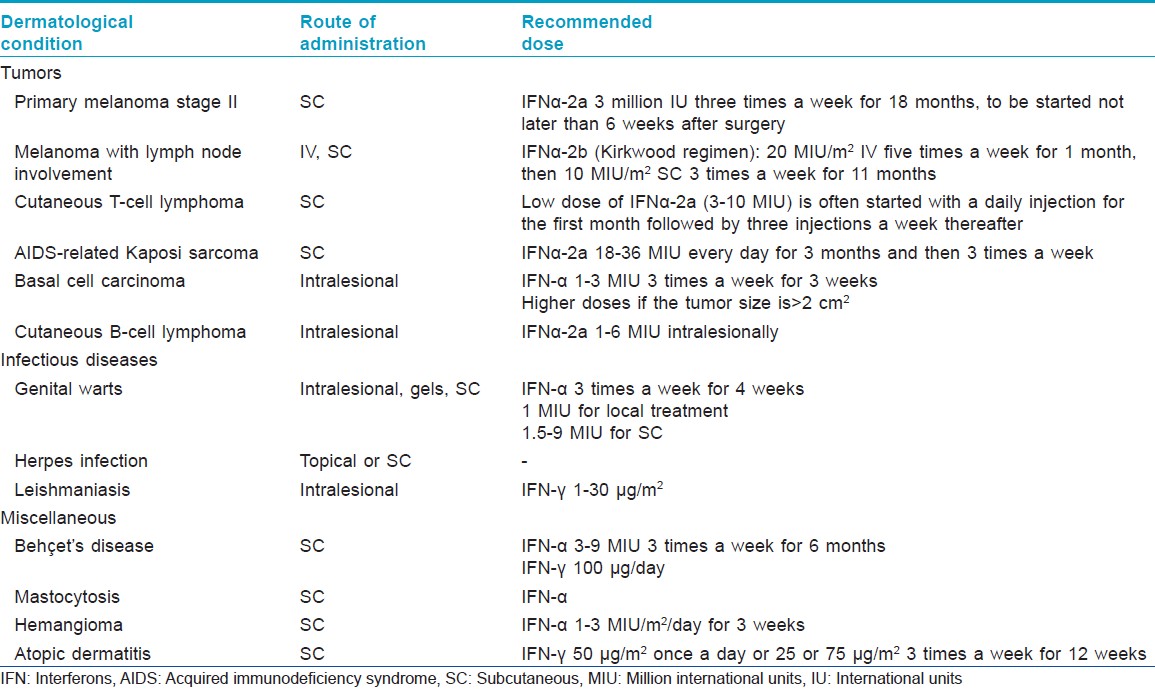
Melanoma [2],[3]For primary melanoma stage II, low-dose subcutaneous IFN-α2a has been licensed as adjuvant treatment. High doses of interferon-α2b are recommended for lymph node involvement according to the Kirkwood study. However, subsequent studies using high dose, intermediate dose, or low dose yielded conflicting results, making it difficult to recommend a specific dose or treatment duration.
Various studies show a significant increase in progression-free survival with interferon in melanoma. [3] Most studies did not report a statistically significant difference in terms of overall survival between the treated and untreated patients. [3] Various good prognostic factors put forth include primary tumor ulceration and development of autoimmunity. [4]
Interferon-α is no longer indicated in patients with distant metastases in view of the poor response rates. [5] Interferon-γ and β are currently under trial.
Cutaneous T-cell lymphoma [6]Interferon-α2a has been used in cutaneous T-cell lymphomas, widespread lymphomatoid papulosis, and cutaneous CD30-positive T-cell lymphomas that are refractory to or cannot be treated by conventional therapies. The response rate is about 60%, with a 20% complete remission rate. Peak efficacy is generally reached after >6 months.
Interferons can be combined with other treatments such as PUVA and extracorporeal photopheresis with higher chances of remission.
Kaposi sarcoma [7]Interferon-α2a is used for the treatment of AIDS-related Kaposi sarcoma with a CD4 count greater than 250/mm 3 and no history of opportunistic infections or constitutional symptoms. The response rates are highly variable and depend upon patient-related factors such as CD4 count. Anti-retroviral treatments such as zidovudine and didanosine act synergistically with interferons, allowing use of lower doses of the latter.
Genital warts
A meta-analysis conducted in 2009 explored the use of local (intralesional injections or gels) or systemic (subcutaneous) interferons versus placebo for genital warts. [8] Complete response rate was significantly higher (P < 0.001) with local IFN (44.4%) than with placebo (16.1%). The risk of recurrence was significantly lower in the local IFN group.
Leishmaniasis [9]
Interferon-γ has been used successfully in leishmaniasis due to its stimulatory effect on macrophages and NK cells with success rates of 70% in the oriental form and 40% in the American form.
Herpes infection [10]
In herpes virus infections, presently, interferons are considered inferior to antiviral agents due to the lower efficacy, higher cost, and poor tolerability.
Behçet′s disease
High response rates with subcutaneous interferon-α have been reported in mucocutaneous lesions (86%), articular lesions (96%), and eye damage refractory to conventional treatments (94%) in Behçet′s disease. [11],[12] Interferon-γ has a beneficial role only against cutaneous manifestations of Behçet′s disease.
Mastocytosis
Subcutaneous interferon-α is one of the first-line treatments for aggressive systemic mastocytosis and is effective against the dermatological, hematological, gastrointestinal, and skeletal symptoms. [13]
Basal cell carcinoma [14]
Various studies report complete response rates of 67-86% with interferon-α in basal cell carcinoma (BCC). Its combination with imiquimod has shown better efficacy. However, it is not effective against aggressive forms such as desmoplastic BCC.
Hemangioma
Interferon-α may be tried in cases where beta-blockers and corticosteroids have failed. [15] The improvement is generally rapid with response rate of about 80%. The main adverse event observed was the rare, irreversible neurotoxicity of the spastic diplegia type.
Atopic dermatitis
IFN-γ has immunomodulating properties due to its inhibitory effect on the production of IL-4. [16] Various studies have shown response rates of approximately 45% in atopic dermatitis patients, irrespective of the dose administered.
Adverse Effects
The adverse effects of interferons are dose-dependent and generally remit either during continued therapy or after dose reduction. The most common side effect reported is "flu-like" symptoms characterized by fever, sweats, chills, myalgias, and arthralgias. These symptoms typically resolve over the first 10 days of treatment and can be managed with paracetamol. Other side effects include hematological (neutropenia, thrombocytopenia), metabolic (hypocalcemia, hyperlipidemia), neurological (headaches, difficulties in concentration, confusion), psychiatric (depression, insomnia, anxiety, mood swings), cardiovascular (palpitations, conduction disturbances, decompensation of unstable cardiac disease), gastrointestinal (nausea, diarrhea, abdominal pain), renal toxicity, cutaneous (reversible alopecia, xerosis, injection site reactions, and pain), and sexual disturbances in the form of menstrual irregularities and decreased libido.
Uncommon side effects include anemia, lymphocytopenia, rhabdomyolysis, convulsions, suicidal ideation, aggravation of psoriasis, urticaria, Raynaud′s syndrome, acrocyanosis, and autoimmune disorders like thyroid dysfunction, systemic lupus erythematosus, rheumatoid arthritis, and leukocytoclastic vasculitis.
Most of these side effects are reversible upon discontinuation, with the exception of certain autoimmune diseases.
The main absolute contraindications are known hypersensitivity to the drug or its components, severe cardiac, renal or hepatic disease, bone marrow failure, uncontrolled epilepsy, and severe psychiatric disorders. Interferons are pregnancy category C drugs with unknown safety during pregnancy and lactation.
Monitoring of Treatment
Laboratory testing
- Complete blood count with differentials including platelet count
- Blood chemistries, including electrolytes and creatine phosphokinase
- Liver function tests [aspartate transaminase (AST), alanine transaminase (ALT)]
- Renal function tests [blood urea nitrogen (BUN), S. creatinine].
Special testing
- ECG is recommended in patients with preexisting cardiac disease
- Thyroid function tests and thyroid autoantibodies like thyroid peroxidase, thyroglobulin, and thyroid stimulating hormone receptor antibodies are recommended on a yearly basis.
Monitoring frequency
- Prior to start of treatment
- Two weeks after initiation of therapy
- Monthly thereafter while on therapy.
Drug Interactions
Drugs that increase the hematological toxicity of interferons are angiotensin converting enzyme inhibitors, methotrexate, zidovudine, cidofovir, and imatinib.
Interferons decrease the clearance of theophylline, thereby increasing its serum levels.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and systemic corticosteroids inhibit the activity of interferons.
Vinca alkaloids (vinblastine, vincristine), when used along with interferons increase the risk of neurotoxicity.
CNS depressants like narcotics, hypnotics, and sedatives increase the risk of neurological adverse effects when used concomitantly with interferons.
CONCLUSION
Interferons have a variety of biological activities. There is an ever-expanding list of their indications in dermatologic diseases. However, it should be kept in mind that interferons are a double-edged sword since they can exert useful anti-inflammatory and immunomodulatory effects while also having the potential to induce or exacerbate autoimmune disorders.
| 1. |
Ealick SE, Cook WJ, Vijay-Kumar S, Carson M, Nagabhushan TL, Trotta PP, et al. Three-dimensional structure of recombinant human interferon-gamma. Science 1991;252:698-702.
[Google Scholar]
|
| 2. |
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: Current status and future prospects. Oncologist 2011;16:5-24.
[Google Scholar]
|
| 3. |
Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: A systematic review and meta-analysis. J Natl Cancer Inst 2010;102:493-501.
[Google Scholar]
|
| 4. |
Eggermont AM, Suciu S, Testori A, Kruit WH, Marsden J, Punt CJ, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: Results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer 2012;48:218-25.
[Google Scholar]
|
| 5. |
Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: A meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol 2007;25:5426-34.
[Google Scholar]
|
| 6. |
Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and sezary syndrome. Blood 2009;114:4337-53.
[Google Scholar]
|
| 7. |
Krown SE. AIDS-associated Kaposi's sarcoma: Is there still a role for interferon alfa? Cytokine Growth Factor Rev 2007;18:395-402.
[Google Scholar]
|
| 8. |
Yang J, Pu YG, Zeng ZM, Yu ZJ, Huang N, Deng QW. Interferon for the treatment of genital warts: A systematic review. BMC Infect Dis 2009;9:156-64.
[Google Scholar]
|
| 9. |
Smith DI, Swamy PM, Heffernam MP. Off-label uses of biologics in dermatology: Interferon and intravenous immunoglobulin (part 1 of 2). J Am Acad Dermatol 2007;56:e1-54.
[Google Scholar]
|
| 10. |
Viera MH, Amini S, Huo R, Konda S, Block S, Berman B. Herpes simplex virus and human papillomavirus genital infections: New and investigational therapeutic options. Int J Dermatol 2010;49:733-49.
[Google Scholar]
|
| 11. |
Mendes D, Correia M, Barbedo M, Vaio T, Mota M, Gonçalves O, et al. Behcet's disease-a contemporary review. J Autoimmun 2009;32:178-88.
[Google Scholar]
|
| 12. |
Onal S, Kazokoglu H, Koc A, Akman M, Bavbek T, Direskeneli H, et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behcet uveitis. Arch Ophthalmol 2011;129:288-94.
[Google Scholar]
|
| 13. |
Pardanani A. Systemic mastocytosis in adults: 2011 update on diagnosis, risk stratification, and management. Am J Hematol 2011;86:362-71.
[Google Scholar]
|
| 14. |
Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: A review. J Am Acad Dermatol 2011;64:413-22.
[Google Scholar]
|
| 15. |
Mabeta P, Pepper MS. Hemangiomas-current therapeutic strategies. Int J Dev Biol 2011;55:431-7.
[Google Scholar]
|
| 16. |
Walling HW, Swick BL. Update on the management of chronic eczema: New approaches and emerging treatment options. Clin Cosmet Investig Dermatol 2010;3:99-117.
[Google Scholar]
|
Fulltext Views
11,072
PDF downloads
6,501





