Translate this page into:
Intermediate doses of rituximab used as adjuvant therapy in refractory pemphigus
Correspondence Address:
Pradnya J Londhe
Opp. Block no. A- 368, Nagsen Nagar, Kurla Camp, Ulhasnagar - 421 004, Maharashtra
India
| How to cite this article: Londhe PJ, Kalyanpad Y, Khopkar US. Intermediate doses of rituximab used as adjuvant therapy in refractory pemphigus. Indian J Dermatol Venereol Leprol 2014;80:300-305 |
Abstract
Background: Rituximab, a monoclonal anti-CD20 antibody, has been used with encouraging results in pemphigus. We describe herein refractory cases of pemphigus vulgaris (n = 23) and pemphigus foliaceus (n = 1) treated with rituximab in addition to steroids and immunosuppressants. Aims: To assess the response to treatment, the duration of clinical remission, serology of the response and adverse effects of rituximab in pemphigus patients. Methods: We recorded observations of 24 patients with pemphigus having either refractory disease in spite of high dose of steroids and immunosuppressants, corticosteroid-dependent disease, strong contraindications to corticosteroids, or severe disease. The patients were treated with infusions of one injection per week for three consecutive weeks of 375 mg of rituximab per m 2 of body-surface area. One similar infusion was repeated after 3 months of 3 rd dose. We observed the clinical outcome after 6 months of 3 rd dose of rituximab and looked for complete healing of cutaneous and mucosal lesions (complete remission). Observations: After follow-up of 7-24 months, five patients showed only partial improvement while 19 of 24 patients had a complete remission 3 months after rituximab. Of these 19 patients, 12 patients achieved complete remission and are off all systemic therapy, and the rest are continuing with no or low dose of steroids with immunosuppressants. Two patients relapsed after initial improvement; one was given moderate dose of oral steroids and immunosuppressant and the other was given repeat single dose of rituximab to control relapse. Conclusion: Rituximab is able to induce a prolonged clinical remission in pemphigus after a single course of four infusions. The high cost and limited knowledge of long term adverse effects are limitations to the use of this biologic agent.INTRODUCTION
Pemphigus is a potentially life-threatening autoimmune blistering disease affecting the skin and mucosa. It is mediated by pathogenic autoantibodies directed against desmoglein 1 and/or desmoglein 3. [1],[2],[3] Current therapies generally suppress the immune system aiming to decrease antibody production. Dexamethasone cyclophosphamide pulse (DCP) therapy or oral corticosteroids with or without adjuvant immunosuppressants (azathioprine, cyclophosphamide, mycophenolate mofetil, and methotrexate) have been used for severe cases of pemphigus in India. [4],[5] However, chronic immune suppression also increases the risk of infections and malignancy. The challenge in pemphigus treatment, therefore, is to balance risk of disease with risk of therapy.
Rituximab, the B-cell depleting anti-CD20 monoclonal chimeric antibody, has been recently introduced as a highly effective rescue medication in refractory cases. It is directed against CD20, a pan B-cell glycoprotein on B lymphocytes from the pre-B cell to the preplasma-cell stage. [6] Pro-B cells, plasmablasts, and plasma cells do not express the CD20 molecule, and are unaffected by rituximab. [7] Among several mechanisms involved in B-cell killing, rituximab exerts B-cell cytolytic activity mainly through antibody-dependent cell-mediated cytotoxicity. [6] The rationale for use of rituximab in patients with pemphigus is based on its ability to deplete CD20+ B cells that presumably produce pathogenic antibodies. [7]
Herein, we present a series of 24 patients with refractory pemphigus treated with intravenous rituximab in addition to our standard line of treatment. We assessed the response to treatment, the duration of clinical remission, serology of the response and adverse effects.
METHODS
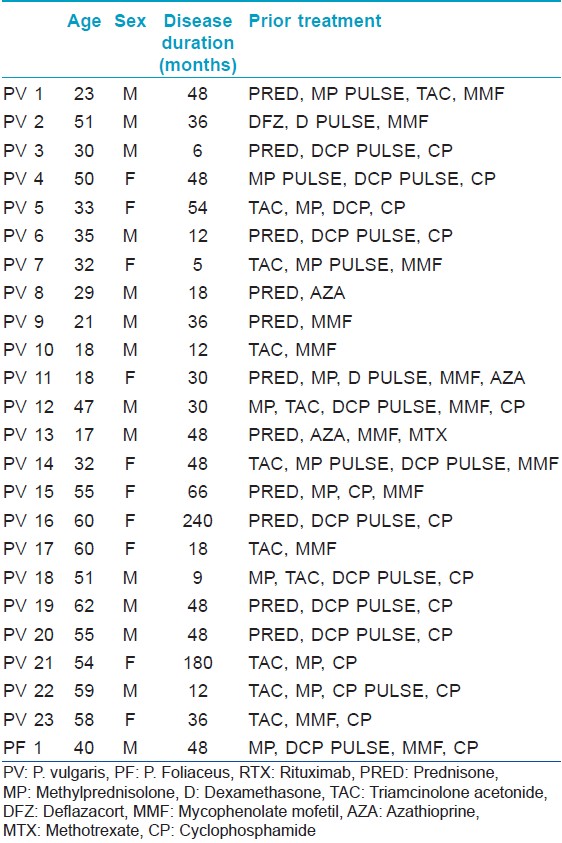
We recorded and compiled the observations of 24 pemphigus patients (23 with pemphigus vulgaris, 1 with pemphigus foliaceus) fulfilling the inclusion criteria, who received rituximab between October 2010 and Feb 2013, with follow-up through September 2013. Diagnoses were confirmed by clinical presentation, histology, and ELISA assays of desmoglein 1 or 3. Inclusion criteria for treatment with rituximab were difficult to treat diseases as per consensus statements for pemphigus. [8] This included refractory disease, steroid dependence, or contraindication to use of conventional therapy or severe disease with unwillingness for conventional therapies and contraindications for use of certain immunosuppressants like cyclophosphamide. All patients had received conventional treatments like dexamethasone cyclophosphamide pulse (DCP), dexamethasone pulse (DP), cyclophosphamide 1-2 mg/kg/day, azathioprine 1-2 mg/kg/day, mycophenolate mofetil 1-2 g/day, methotrexate 10-25 mg/wk and prednisolone 1-1.5 mg/kg/day in the past for periods varying between 5 months and 20 years. Detailed drug history for each patient is given in [Table - 1].
Five patients were refractory to conventional treatment, 9 had steroid dependence, 4 had contraindications to the use of conventional therapy and 5 had severe disease and were unwilling to take conventional therapies. Monitoring of the disease activity was done according to a PAS, pemphigus activity score severity score published by the Herbst and Bystryn. [9] Skin biopsy from all patients was submitted for histological assessment using hematoxylin-eosin staining for all the patients. Serum anti-desmoglein antibody levels were determined. Pre-treatment workup included a complete hemogram, liver function tests, renal function tests, fasting and post-prandial blood sugar levels, chest X-ray, Mantoux test, screening for viral infections including HBsAg, anti-HBcIgM, anti- HCV, HIV-1 and HIV-2, and serum IgG.
Patient with pregnancy, breastfeeding, sensitivity to murine proteins, active hepatitis or HIV infection, widespread infections, cardiac disease, children and patients found positive for above antibodies against infections were excluded.
Patients were premedicated with dexamethasone (8 mg) intravenously 1 hour prior to infusion and pheneramine maleate 22.75 mg intravenously immediately prior to infusion. Pulse and blood pressure were monitored every 15 mins and patients were watched for hypotension, nausea, headache, giddiness, chills, fever, and rash. Drip rates were reduced if infusion related side effects occurred.
Rituximab was administered using a modification of the lymphoma regimen consisting of infusions of one injection per week for three consecutive weeks of 375 mg/m 2 and another such infusion given 3 months after the third infusion. The fourth injection was delayed to reduce chances of septicemia from active and infected lesions of pemphigus.
The first infusion was given at a rate of 50 mg/h with 30-min escalation by 50 mg/h and a maximum infusion rate of 400 mg/h; the total infusion time being 3-4 h given in an ICU setting. Subsequent infusions were given at a rate of 100 mg/h with 30-min escalations and on a day-care basis. Corticosteroids were maintained at the initial dose until the disease was controlled, and the corticosteroid dose was then reduced according to clinical response. Patients were monitored with blood counts, biochemistry and anti-desmoglein 3 and/or 1 levels 3 months after the last dose.
Response was classified as complete remission when all cutaneous and mucosal lesions were completely healed (i.e. extent of disease score, 0 in PAS) irrespective of treatment given, 6 months after the third dose of rituximab. Patients failing to show complete remission but who were responding to therapy were considered to be in partial remission.
Statistical analysis
Data was analyzed using SPSS 16.0 for Windows. It was reported as mean or median wherever appropriate. Wilcoxon signed ranks test was used to assess significant differences in mean PAS at baseline and 3 months after the last dose. Similarly, the median values of anti-Dsg3 were calculated using the same method and at the same duration. A P < 0.05 was considered significant in all calculations.
RESULTS
The study population included 23 (96%) patients with pemphigus vulgaris and 1 (4%) with pemphigus foliaceous, with 14 (58%) men and 10 (42%) women. The mean age was 43.5 yrs (range 17-62yrs). The mean disease duration prior to rituximab treatment was 47. 33 months (range 5-240 months). All patients were on systemic immunosuppressive therapies for a duration of 5-240 months at the time of the first rituximab infusion [Table - 1]. The mean disease severity scores, calculated by pemphigus activity score (PAS) were 5.58 (range 3-9) at baseline falling to 2.04 (0-6) 6 months after the third dose (P < 0.001). After rituximab treatment, patients were followed up for a duration ranging from 7 to 24 months (mean 18 months). All 24 (100%) patients experienced clinical improvement of disease activity. Overall, 19 (79%) patients achieved complete remission of disease, nine out of these 19 patients were off all systemic therapy after a mean duration of 9 months (6-15 months). The other 10 patients had complete remission but were on no or minimal steroids and tapering doses of immunosuppressants. Out of these 10 patients, one relapsed after 6 months. Five (21%) patients had partial remission and were on low dose steroids (up to 20 mg prednisolone/day) and immunosuppressants. Of these, three patients eventually responded to treatment and showed delayed complete remission after a mean duration of 15 months (10-21 months). One of five patients showing partial remission relapsed in the follow-up period after 15 months. The results are summarized in [Figure - 1].
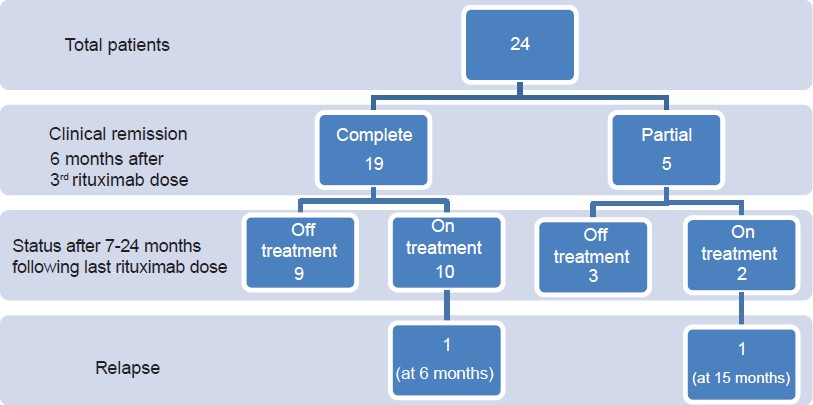 |
| Figure 1: Flow chart of patients showing response to rituximab |
Antibodies against Dsg1 and Dsg3 were measured at the end of the follow-up period in 20 patients. Fifteen patients had their sera analyzed both at the start of therapy and at the end of the follow-up period for anti-Dsg3 (excluding patient with pemphigus foliaceus and patients having baseline values in negative range). Index values of these 20 patients were analyzed. The median of anti-Dsg3 antibody levels in PV patients reduced from 160 at baseline to 4.2 at the end of follow-up period (P = 0.001). The majority of patients showed levels in negative range except for three patients whose anti-Dsg3 levels remained positive. Out of these, anti-Dsg3 levels of two patients eventually became negative and one patient relapsed. The clinical outcome and the antibody levels are summarized in [Table - 2].
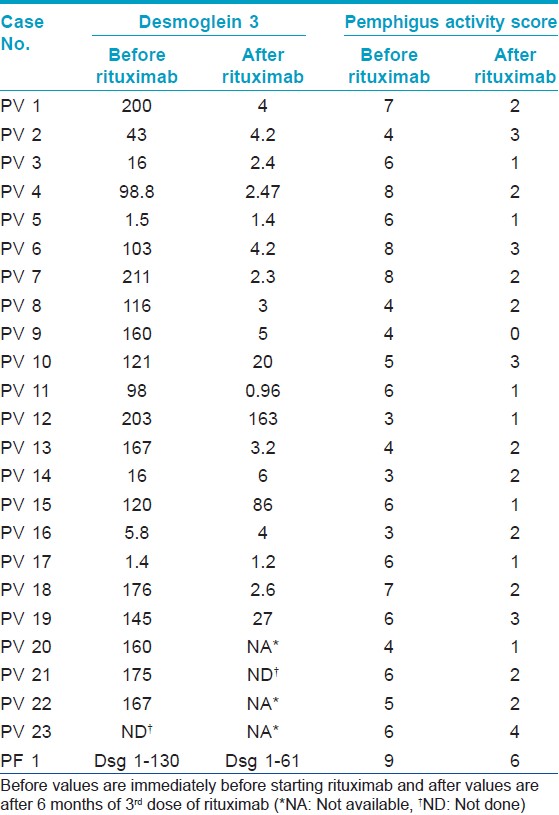
Two patients relapsed after rituximab therapy even while continuing other medications, one with P. foliaceous after 15 months and other with P. vulgaris after 6 months following the last dose of rituximab. Both continued to show positivity for Dsg1 and Dsg 3 respectively. First patient was retreated with two repeat doses of rituximab 3 months apart and is still under observation with partial remission, and the other was treated with moderate dose (40 mg prednisolone) of oral steroids and immunosuppressant and is responding to treatment well.
Side effects occurred mainly during the first infusions, and typically included moderate and brief fever and chills (n = 6), hypotension (n = 2), and hypertension (n = 1) which were controlled by either stopping or decreasing the rate of infusion. By the second and subsequent infusions, the majority of patients experienced no further infusion-related toxicities. No other acute complications were noted. Two (8%) patients developed herpes zoster and the next infusion was deferred till the lesions healed after treatment with an antiviral drug. One of the two also developed a extensive tinea corporis and was treated appropriately. A diabetic patient developed carbuncle that was treated with appropriate antibiotics. He later developed pulmonary embolism (a month following the first three infusions) and was treated successfully in an ICU setting. One patient developed recurrent diarrhea with weight loss of 10 kg within a month and was diagnosed as Isospora diarrhea that was treated successfully with cotrimoxazole.
DISCUSSION
Pemphigus is a relatively common autoimmune mucocutaneous blistering disorder in the Asian sub-continent. In India, pemphigus is seen in a younger age group and is severe as compared to Western countries. Pemphigus vulgaris is the most common subtype accounting for nearly 75-92% of pemphigus cases. [5] Indian patients usually show a good response to conventional dexamethasone-cyclophosphamide pulse and oral corticosteroids, compared to other population groups. However, some patients are resistant to conventional therapy or have contraindications for their use or become steroid dependant. We used rituximab therapy in such patients.
In a recent review by Zakka et al.[10] , rituximab was noted to have been used in the following ways: (1) lymphoma protocol, (2) rheumatoid arthritis protocol, and (3) modifications or different combinations of either protocol. The lymphoma protocol consists of four weekly infusions of 375 mg/m 2 . The rheumatoid arthritis protocol consists of two infusions of 1,000 mg, 2 weeks apart. Forty-two studies 272 patients were found, with 180 were treated by the lymphoma protocol and 92 by the rheumatoid arthritis protocol. Both protocols were effective in treating recalcitrant pemphigus vulgaris. A complete remission occurred in 66.7% of patients on the lymphoma protocol and 75% in the rheumatoid arthritis protocol. A recent meta-analysis encompassing 153 pemphigus patients reported a complete remission rate of 65% after rituximab therapy. [7]
Our observations demonstrated the efficacy of rituximab for pemphigus using a modified lymphoma protocol; 79% of patients were in complete remission 3 months after receiving four infusions of rituximab. We used intermediate dosages (0.5 g × 3 weekly doses and a booster at three months) in order to reduce side effects. After a mean duration of 18 months of follow-up, 22 patients were free of disease and 12 of these patients were not receiving any systemic therapy. Two relapses were treated with, repeat rituximab infusion in one and increased dose of steroid and immunosuppressants in the other. There was correlation of the titre of Dsg 1 and Dsg 3 with disease activity in both these patients but no correlation was found with other comorbidities like infection. The patients who showed a very good response, 12 had been given DCP and 11 had been given high-dose steroids. Overall, treatment with rituximab resulted both in major clinical improvement and a large decrease in the doses of corticosteroids and immunosuppressants.
Most cases were of pemphigus vulgaris (23 out of 24) and skin lesions correlated with Dsg 3 levels. There was a 82.8% fall in the mean antibody levels of Dsg3. Following treatment, the majority of patients showed values in negative ELISA range except for three patients whose Dsg3 levels remained positive. Dsg 3 levels corresponded with clinical activity as one of the patient showing positive value after rituximab therapy developed a relapse.
Use of rituximab is associated with adverse effects. In a study by Joly et al., two (10%) of 21 patients with pemphigus experienced severe infections, including fatal septicemia. [11] A meta-analysis of 153 pemphigus patients treated with rituximab showed that 7% developed serious infections, with 2 (1.3%) fatalities. [7] In our study, nine patients developed mild infusion reaction during their first infusion, subsequent infusions being uneventful. These reactions tended to resolve on slowing infusion rates or addition of corticosteroids, antipyretics, and antihistamines and did not recur in subsequent infusions. [12]
One (4%) of our patients experienced a serious adverse event, namely pulmonary embolism, probably attributable to rituximab 1 month after the 3 rd infusion. In one series, one patient developed deep venous thrombosis 12 weeks following rituximab therapy. This patient subsequently developed a pulmonary embolus. [13] Another patient developed a pulmonary embolism 10 weeks post-rituximab therapy following community-acquired pneumonia. [14]
Serious life-threatening infections did not occur in our patients. Two patients (8%) developed herpes zoster, one developed extensive tinea corporis and another developed Isospora diarrhea leading to significant weight loss. This patient was also on concurrent DCP therapy for 2 years and oral steroids, and the case details are being separately published. Late adverse effects have been described with late onset neutropenia reported 3-23 weeks after rituximab infusion. [15],[16] However, none of our patients developed this side effect.
We observed symptoms of hypothalamo-pituitary-adrenal axis suppression and hypoadrenalism in four patients due to rapid taper of steroids that was possible after rituximab. This necessitated continuation of low dose steroid (5-10 mg of prednisolone) to prevent hypoadrenalism. We also observed transient vesicular lesions, mostly in the oral cavity but sometimes on skin, in a few patients who were off all systemic therapy. The lesions used to heal on their own within 2-3 days. Dsg levels in a majority of these patients were negative making it difficult to be sure about the nature of these transient lesions. None of these lesions could be biopsied for confirmation. This finding has not been described in previous studies.
In conclusion, rituximab is able to induce a prolonged clinical remission in pemphigus. The high cost limited the use even in eligible candidates. Infusion related reactions are known to occur but were limited to the first infusion; hence we could give the subsequent doses on day-care basis in our hospital. This is especially applicable to a resource poor setting like India. High cost and limited knowledge of long-term adverse effects remain the limitations to the use of this biologic agent.
| 1. |
Stanley JR. Pemphigus and pemphigoid as paradigms of organ-specific, autoantibody- mediated diseases. J Clin Invest 1989;83:1443-8.
[Google Scholar]
|
| 2. |
Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol 1993;53:291-325.
[Google Scholar]
|
| 3. |
Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell 1991;67:869-77.
[Google Scholar]
|
| 4. |
Kaur S, Kanwar AJ. Dexamethasone-cyclophosphamide pulse therapy in pemphigus. Int J Dermatol 1990;29:371-4.
[Google Scholar]
|
| 5. |
Kanwar AJ, De D. Pemphigus in India. Indian J Dermatol Venereol Leprol 2011;77:439-49.
[Google Scholar]
|
| 6. |
Schmidt E, Brocker EB, Goebeler M. Rituximab in treatment-resistant autoimmune blistering skin disorders. Clin Rev Allergy Immunol 2008;34:56-64.
[Google Scholar]
|
| 7. |
Feldman RJ, Ahmed AR. Relevance of rituximab therapy in pemphigus vulgaris: Analysis of current data and the immunologic basis for its observed responses. Expert Rev Clin Immunol 2011;7:529-41.
[Google Scholar]
|
| 8. |
Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol 2008;58:1043-6.
[Google Scholar]
|
| 9. |
Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol 2000;42:422-7.
[Google Scholar]
|
| 10. |
Zakka LR, Shetty SS, Ahmed AR. Rituximab in the treatment of pemphigus vulgaris. Dermatol Ther (Heidelb) 2012;2:17.
[Google Scholar]
|
| 11. |
Joly P, Mouquet H, Roujeau JC, D'Incan M, Gilbert D, Jacquot S, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007;357:545-52.
et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007;357:545-52.'>[Google Scholar]
|
| 12. |
Vogel WH. Infusion reactions: Diagnosis, assessment, and management. Clin J Oncol Nurs 2010;14:E10-21.
[Google Scholar]
|
| 13. |
Shimanovich I, Nitschke M, Rose C, Grabbe J, Zillikens D. Treatment of severe pemphigus with protein A immunoadsorption, rituximab and intravenous immunoglobulins. Br J Dermatol 2008;158:382-8.
[Google Scholar]
|
| 14. |
Schmidt E, Seitz CS, Benoit S, Brocker EB, Goebeler M. Rituximab in autoimmune bullous diseases: Mixed responses and adverse effects. Br J Dermatol 2007;156:352-6.
[Google Scholar]
|
| 15. |
Voog E, Morschhauser F, Solal-Celigny P. Neutropenia in patients treated with rituximab. N Engl J Med 2003;348:2691-4.
[Google Scholar]
|
| 16. |
Wolach O, Bairey O, Lahav M. Late-onset neutropenia after rituximab treatment: Case series and comprehensive review of the literature. Medicine (Baltimore) 2010;89:308-18.
[Google Scholar]
|
Fulltext Views
4,354
PDF downloads
2,385





