Translate this page into:
Intralesional drug therapy in dermatology
Correspondence Address:
Nitika S Deshmukh
Department of Skin and VD, B. J. G. Medical College, Pune - 411 001, Maharashtra
India
| How to cite this article: Deshmukh NS, Belgaumkar VA, Mhaske CB, Doshi BR. Intralesional drug therapy in dermatology. Indian J Dermatol Venereol Leprol 2017;83:127-132 |
Introduction
Intralesional therapy is the injection of a higher concentration of a drug directly into skin lesions without significant systemic absorption. The rationale for this technique is the establishment of a subepidermal depot which bypasses the superficial barrier zone.[1]
The different drugs used for intralesional therapy, along with the indications for their use are given below.
Corticosteroids [Table - 1][1]

Cortisone and hydrocortisone acetate suspensions were used from the early 1950s before introduction of preparations with lower solubility such as triamcinolone acetonide, which is now the most common agent used.[2] Triamcinolone acetonide (2.5–10 mg/ml [1 mg/cm [2]]), betamethasone sodium phosphate/acetate (1–2 mg/cm [2]/site), dexamethasone acetate (0.8–1.6 mg/site), dexamethasone sodium phosphate (170 µg–5 mg/session), hydrocortisone acetate (5–7.5 mg/session) and methylprednisolone acetate (20–60 mg/session) are used for keloids, alopecia areata, nodulocystic acne, granuloma annulare, granuloma faciale, hidradenitis suppurativa, localized and nail psoriasis, prurigo nodularis, cutaneous lupus erythematosus, vitiligo, lichen simplex chronicus, hypertrophic nail lichen planus, resistant oral pemphigus, hemangiomas, etc.[1] The dose per se ssion is generally 0.1–0.2 ml/cm [2] of involved skin (<1–2 ml/dose) with an interval of 3–6 weeks between two consecutive injections. The number of injections depends on the disease, site of lesions, age of the patient and response to previous injections. The maximum dosage of triamcinolone acetonide should not exceed 40 mg/ml/session. Corticosteroids can be injected in full strength or diluted with normal saline or local anesthetic.[2]
Side effects
Atrophy, pain, hyper- or hypopigmentation, telangiectasias, striae, hirsutism, secondary infection, stellate pseudoscars, hypertrichosis, amaurosis fugax and tachyphylaxis could be possible side-effects of intralesional corticosteroid injections.
5-Fluorouracil
[Table - 2].[3],[4],[5],[6] enlists the conditions for which intralesional 5-fluorouracil (available as 50 mg/ml ampoule) is used.
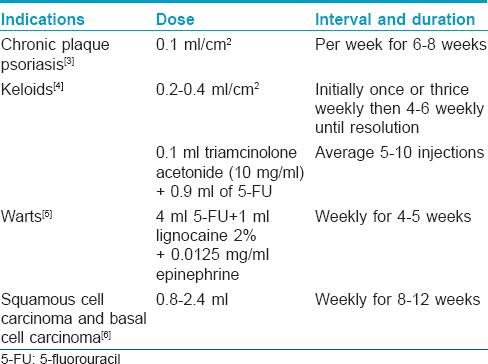
Side effects
Pain, necrosis, hyperpigmentation and atrophic scarring.
Fillers, Botulinum Toxin a and Platelet Rich Plasma
Platelet-rich plasma has been used, with promising results, at weekly intervals, for alopecia areata, acne scar revision, non-healing ulcers, lipodermatosclerosis, striae distensae, androgenetic alopecia, lichen sclerosus and vitiligo.[7]
Botulinum toxin A and hyaluronic acid fillers have been used successfully for the treatment of traumatic scars, facial rejuvenation [Table - 3] correction of contour defects and photoaging.[8]
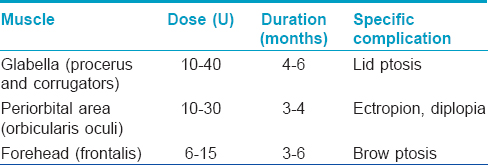
Botulinum toxin A is also used in the treatment of hyperhidrosis in a dose of 50–200U injected intradermally (the full dose being divided into 10–15 aliquots at spatial intervals of 2 cm). It reduces sweat production for 3–17 months in palmoplantar and axillary hyperhidrosis. Injections may be repeated after 6 months, if required.[9]
Side effects
Autologous platelet rich plasma is safe with no risk of hypersensitivity. Botulinum toxin and fillers can cause edema, pain, erythema, temporary hypoesthesia and over- or under- correction.
Interferons
Use of intralesional interferons in various dermatological indications is shown in [Table - 4].[10]
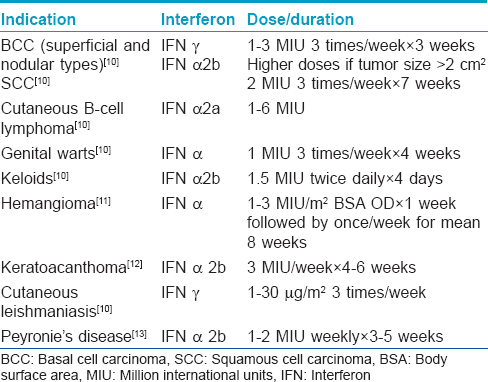
Side effects
Flu-like symptoms, pancytopenia, hypocalcemia, hyperlipidemia, depression, cardiac arrhythmias, gastrointestinal upset, renal toxicity, alopecia, xerosis, injection site reactions and menstrual irregularities can all be seen.
Mesotherapy
Mesotherapy is a semi-invasive method of drug delivery that consists of multiple dermal or subcutaneous injections of mixture of compounds in minute doses. It is actually a “intra-dermotherapy” instead of an “intralesional therapy”. In intralesional therapy, the injection is targeted inside the skin condition to be treated irrespective of whether the skin lesion is in dermis or subcutis. But in mesotherpy, the depth of penetration of needle should not exceed more than 4 mm into the skin and injections are regularly spaced.[14]
Mesotherapy with various drugs is useful for androgenic alopecia and telogen effluvium (biotin, minoxidil and dutasteride), cellulite lipolysis and localized fat dissolution (phosphatidylcholine, vitamin complex, trace elements, collagenases, hyaluronidases, etc.). Meglumine antimoniate (by mesogun) is used for cutaneous leishmaniasis.[14]
Side effects
Side effects observed include pain, edema, erythema, local infection or abscess, lichenoid eruptions, hyperpigmentation and hypersensitivity reactions.
Cryotherapy
Intralesional cryotherapy with liquid nitrogen has been used, together with steroids, for the treatment of keloids and hypertrophic scars.[15]
Side effects
Pain, erythema, hypo- or hyperpigmentation at the site of injections are seen.
Sclerosants
Sodium tetradecyl sulfate (1% and 3%), hypertonic saline, polidocanol, sodium morrhuate, polyiodinated iodine and chromated glycerin are used in pyogenic granuloma, superficial telangiectasias, venulectasias of lower extremities, hemangioma, lymphangioma circumscriptum and venous malformations in a dose of 0.1 to 0.5 ml/site at weekly intervals.[1]
Side effects
Nicolau syndrome, which manifests with tissue necrosis, was reported with sclerotherapy by Nirmal et al., in a single case of pyogenic granuloma.[16] Other complications include pain, ecchymosis, hyperpigmentation, necrosis, ulceration and thrombophlebitis.
Bleomycin
Although not Food and Drug Administration approved for intralesional therapy, bleomycin has been used in keloids (1.5 IU/ml or 0.1–1 ml/monthly), periungual warts (0.1–2 ml/session, monthly, up to 4 injections/wart), large hemangiomas and venous malformations (0.3–1 mg/kg, up to a maximum of 15 mg/month), keratoacanthomas, cutaneous malignancies and cutaneous leishmaniasis.[17],[18],[19],[20],[21] It is available as bleomycin sulfate powder lyophilized 15U/vial (reconstituted with 1–5 ml sterile water for injection or 0.9% normal saline) or 30U/vial (2–10 ml water/normal saline). It requires storage at 2–8°C. Shelf life is 24 h at room temperature and 4 weeks if refrigerated.[18] Bleomycin penetrates poorly through cell membranes. Reconstitution in local anesthetic (lignocaine) improves penetration by disrupting cell membranes.
Side effects
Since intralesional injection is extremely painful, it is given under infiltrative local anesthesia. Jet injectors, modified tattoo machines, monolet needles and pricking through bifurcated needles reduce procedural pain. Redness, swelling, pain and burning subside after 72 h. Rare side effects are Raynaud's phenomenon, narrowing of fingertips, restricted nail growth, scarring, lymphangitis, paresthesias and hematoma formation.
Sodium Stibogluconate Antimony
It is a first line therapy in localized cutaneous leishmaniasis in a dose of 50 mg/cm [2]/week for 5–7 weeks.[22]
Side effects
Pain and hyperpigmentation may sometimes be seen.
Amphotericin B
It has been successfully tried in lesions of cutaneous leishmaniasis in a dose of 2 mg/ml/week, intralesionally for up to 12 weeks with an average of 10.31 ± 5.41 injections.[23]
Side effects
Pain, fibrosis, local allergic reaction and systemic absorption are possible side effects.
Methotrexate
In a recent study, Yoo and Kim reported complete resolution of keratoacanthomas with 2–7 injections (mean 4.3) of methotrexate, 25 mg/ml with an average total dose of 53.7 mg/treatment course [24] and an average injection interval of 10 days (range 4–28 days). Other indications where intralesional methotrexate has been tried with variable success are cutaneous malignancies such as malignant melanoma, basal cell carcinoma and squamous cell carcinoma, as well as in nail psoriasis.[20],[25]
Side effects
Side effects observed have been ulceration and necrosis, and pancytopenia (after systemic absorption).
Vincristine and Vinblastine
These cytotoxic agents are used in Kaposi's sarcoma.[26],[27] They act by blocking microtubule polymerization after binding with tubulin, arresting cell division thus leading to cell death.
Vinblastine is available in 10 ml vials and is used in a dose of 1 mg/ml. About 0.03–0.1 ml of the drug is injected after diluting with 0.9% normal saline. Vincristine is injected in volumes of 0.03–0.08 ml of a 1 µg/ml solution.[26],[27]
Side effects
Pain, erythema and pruritus have been noted.
Immunotherapy
This mode of therapy utilizes the ability of the immune system to mount delayed type hypersensitivity to various injected antigens and wart or tumor tissue. Older individuals (>40 years) are less likely to respond, due to age-related blunting of immune responses. Different vaccines and antigens have been used with variable results as summarized in [Table - 5].[28],[29],[30]
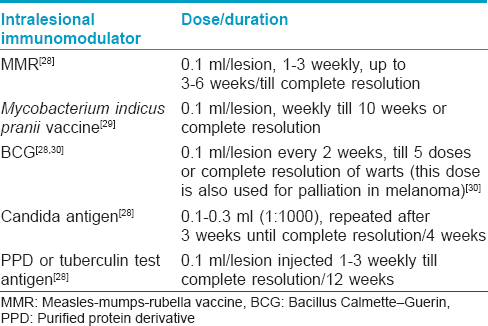
Other antigens such as trichophyton skin test antigen have also been used with varied success in the treatment of warts.[28]
Side effects
Pain, pruritus, chills, myalgia and arthralgia may be noted.
Verapamil
Margaret Shanthi et al. reported a reduction in vascularity, pliability and dimensions of keloids and hypertrophic scars after intralesional verapamil, 2.5 mg/ml every 3 weeks for 6 months.[31] It has also been tried in the prevention and treatment of Peyronie's disease.[32] No side effects have been noted except for rare injection site reactions.
Photodynamic Therapy
Photosensitizers like aminolevulinic acid have been injected intralesionally and followed by irradiation with a good response in cutaneous malignancies (nodular basal cell carcinoma, squamous cell carcinoma), acne and hidradenitis suppurativa.[6],[33],[34],[35]
Side effects
Injection site reactions may occur.
Rituximab
This anti-CD20 monoclonal antibody was tried successfully in primary cutaneous B-cell lymphoma (10 mg/ml [3 ml]) 3 times/week followed by a 3-week treatment-free period with acceptable results after nine injections.[36]
Side effects
Injection site reactions may be noted.
Intralesional Cyclosporine
Ho et al., documented statistically significant improvement in chronic plaque psoriasis with 17 mg/ml injected 3 times/week for 4 weeks.[37]
Side effects
Injection site reactions may be seen.
Mistletoe Extract
It is a whole plant remedy derived from Viscum album L., a hemiparasite shrub; and is used in a dose of 20 ml/month [20 mg/ml/ampoule]. Two case reports mention its intralesional use in primary cutaneous B-cell lymphoma with complete remission in 8–12 months.[38]
Side effects
Injection site reactions may be noted.
Advantages of Intralesional Therapy
- Faster action
- Prolonged duration of action due to depot/reservoir effect
- Eliminates need for long-term topical therapy and avoids side effects of systemic treatment
- Improves patient compliance
- Penetrates deeper than topical therapy
- Can be combined with other modalities for synergistic action. For example, intralesional drugs with cryotherapy for keloids.
Contraindications for Intralesional Therapy
- Active impetigo or herpetic infection at injection site
- Previous history of hypersensitivity.
Conclusion
When used cautiously and judiciously, intralesional therapy is a simple, fairly safe office procedure constituting an integral part of the dermatological therapeutic armamentarium.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Savant S, editor. Intralesional therapy. In: Textbook of Dermatosurgery and Cosmetology. 2nd ed. Mumbai, India: ASCAD; 2005. pp 100-106.
[Google Scholar]
|
| 2. |
Verbov J. The place of intralesional steroid therapy in dermatology. Br J Dermatol 1976;94 Suppl 12:51-8.
[Google Scholar]
|
| 3. |
Mahajan BB, Singla M. Evaluation of intralesional 5% 5-fluorouracil in resistant localized plaque psoriasis. Indian Dermatol Online J 2014;5:287-90.
[Google Scholar]
|
| 4. |
Mutalik S. Treatment of keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol 2005;71:3-8.
[Google Scholar]
|
| 5. |
Yazdanfar A, Farshchian M, Fereydoonnejad M, Farshchian M. Treatment of common warts with an intralesional mixture of 5-fluorouracil, lidocaine, and epinephrine: A prospective placebo-controlled, double-blind randomized trial. Dermatol Surg 2008;34:656-9.
[Google Scholar]
|
| 6. |
Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: A review. J Am Acad Dermatol 2011;64:413-22.
[Google Scholar]
|
| 7. |
Arshdeep K, Kumaran MS. Platelet-rich plasma in dermatology: Boon or a bane? Indian J Dermatol Venereol Leprol 2014;80:5-14.
[Google Scholar]
|
| 8. |
Nanda S, Bansal S. Upper face rejuvenation using botulinum toxin and hyaluronic acid fillers. Indian J Dermatol Venereol Leprol 2013;79:32-40.
[Google Scholar]
|
| 9. |
Salmanpoor R, Rahmanian MJ. Treatment of axillary hyperhidrosis with botulinum-A toxin. Int J Dermatol 2002;41:428-30.
[Google Scholar]
|
| 10. |
Mahajan BB, Kaur S. Interferons. Indian J Dermatol Venereol Leprol 2015;81:51-5.
[Google Scholar]
|
| 11. |
Kaselas C, Tsikopoulos G, Papouis G, Kaselas V. Intralesional administration of interferon A for the management of severe haemangiomas. Pediatr Surg Int 2007;23:215-8.
[Google Scholar]
|
| 12. |
Oh CK, Son HS, Lee JB, Jang HS, Kwon KS. Intralesional interferon alfa-2b treatment of keratoacanthomas. J Am Acad Dermatol 2004;51 5 Suppl: S177-80.
[Google Scholar]
|
| 13. |
Lacy GL 2nd, Adams DM, Hellstrom WJ. Intralesional interferon-alpha-2b for the treatment of Peyronie's disease. Int J Impot Res 2002;14:336-9.
[Google Scholar]
|
| 14. |
Konda D, Thappa DM. Mesotherapy: What is new? Indian J Dermatol Venereol Leprol 2013;79:127-34.
[Google Scholar]
|
| 15. |
Goldenberg G, Luber AJ. Use of intralesional cryosurgery as an innovative therapy for keloid scars and a review of current treatments. J Clin Aesthet Dermatol 2013;6:23-6.
[Google Scholar]
|
| 16. |
Nirmal B, Segu SS, Sacchidanand SA, Deshpande P. Nicolau syndrome following sclerotherapy for pyogenic granuloma. Indian J Dermatol Venereol Leprol 2014;80:484.
[Google Scholar]
|
| 17. |
Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician 2009;80:253-60.
[Google Scholar]
|
| 18. |
Amarnani AN. Treatment of warts. In: Arndt KA, Jeffery TS, Alam M, Bhatia A, Chilukuri S, editor. Manual of Dermatologic Therapeutics. 8th ed. New Delhi, India: Wolters Kluwer, Pvt. Ltd.; 2015. p. 362-6.
[Google Scholar]
|
| 19. |
Pienaar C, Graham R, Geldenhuys S, Hudson DA. Intralesional bleomycin for the treatment of hemangiomas. Plast Reconstr Surg 2006;117:221-6.
[Google Scholar]
|
| 20. |
Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: A practical review. J Am Acad Dermatol 2010;63:689-702.
[Google Scholar]
|
| 21. |
Saitta P, Krishnamurthy K, Brown LH. Bleomycin in dermatology: A review of intralesional applications. Dermatol Surg 2008;34:1299-313.
[Google Scholar]
|
| 22. |
Bumb RA, Mehta RD, Ghiya BC, Jakhar R, Prasad N, Soni P, et al. Efficacy of short-duration (twice weekly) intralesional sodium stibogluconate in treatment of cutaneous leishmaniasis in India. Br J Dermatol 2010;163:854-8.
[Google Scholar]
|
| 23. |
Goyonlo VM, Vosoughi E, Kiafar B, Nahidi Y, Momenzadeh A, Taheri AR. Efficacy of intralesional amphotericin B for the treatment of cutaneous leishmaniasis. Indian J Dermatol 2014;59:631.
[Google Scholar]
|
| 24. |
Yoo MG, Kim IH. Intralesional methotrexate for the treatment of keratoacanthoma: Retrospective study and review of the korean literature. Ann Dermatol 2014;26:172-6.
[Google Scholar]
|
| 25. |
Saricaoglu H, Oz A, Turan H. Nail psoriasis successfully treated with intralesional methotrexate: Case report. Dermatology 2011;222:5-7.
[Google Scholar]
|
| 26. |
Boudreaux AA, Smith LL, Cosby CD, Bason MM, Tappero JW, Berger TG. Intralesional vinblastine for cutaneous Kaposi's sarcoma associated with acquired immunodeficiency syndrome. A clinical trial to evaluate efficacy and discomfort associated with infection. J Am Acad Dermatol 1993;28:61-5.
[Google Scholar]
|
| 27. |
Odom RB, Goette DK. Treatment of cutaneous Kaposi's sarcoma with intralesional vincristine. Arch Dermatol 1978;114:1693-4.
[Google Scholar]
|
| 28. |
Sinha S, Relhan V, Garg VK. Immunomodulators in warts: Unexplored or ineffective? Indian J Dermatol 2015;60:118-29.
[Google Scholar]
|
| 29. |
Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol 2014;80:509-14.
[Google Scholar]
|
| 30. |
Mastrangelo MJ, Sulit HL, Prehn LM, Bornstein RS, Yarbro JW, Prehn RT. Intralesional BCG in the treatment of metastatic malignant melanoma. Cancer 1976;37:684-92.
[Google Scholar]
|
| 31. |
Margaret Shanthi FX, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol 2008;74:343-8.
[Google Scholar]
|
| 32. |
Levine LA. Treatment of Peyronie's disease with intralesional verapamil injection. J Urol 1997;158:1395-9.
[Google Scholar]
|
| 33. |
Kim SK, Shin J, Kim YC. Nodular basal cell carcinoma successfully treated with intralesional 5-aminolevulinic acid injection-photodynamic therapy. J Dermatol 2012;39:254-5.
[Google Scholar]
|
| 34. |
Ryou JH, Lee SJ, Park YM, Kim HO, Kim HS. Acne-photodynamic therapy with intra-lesional injection of 5-aminolevulinic acid. Photodermatol Photoimmunol Photomed 2009;25:57-8.
[Google Scholar]
|
| 35. |
Valladares-Narganes LM, Rodríguez-Prieto MA, Blanco-Suárez MD, Rodriguez-Lage C, García-Doval I. Treatment of hidradenitis suppurativa with intralesional photodynamic therapy using a laser diode attached to an optical cable: A promising new approach. Br J Dermatol 2015;172:1136-9.
[Google Scholar]
|
| 36. |
Heinzerling L, Dummer R, Kempf W, Schmid MH, Burg G. Intralesional therapy with anti-CD20 monoclonal antibody rituximab in primary cutaneous B-cell lymphoma. Arch Dermatol 2000;136:374-8.
[Google Scholar]
|
| 37. |
Ho VC, Griffiths CE, Ellis CN, Gupta AK, McCuaig CC, Nickoloff BJ, et al. Intralesional cyclosporine in the treatment of psoriasis. A clinical, immunologic, and pharmacokinetic study. J Am Acad Dermatol 1990;22:94-100.
[Google Scholar]
|
| 38. |
Orange M, Lace A, Fonseca MP, von Laue BH, Geider S, Kienle GS. Durable regression of primary cutaneous B-cell lymphoma following fever-inducing mistletoe treatment: Two case reports. Glob Adv Health Med 2012;1:18-25.
[Google Scholar]
|
Fulltext Views
31,817
PDF downloads
8,628





