Translate this page into:
Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts
Correspondence Address:
Somesh Gupta
Department of Dermatology, Venereology, and Leprology, All India Institute of Medical Sciences, New Delhi - 110 029
India
| How to cite this article: Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts . Indian J Dermatol Venereol Leprol 2014;80:509-514 |
Abstract
Background: Multiple cutaneous warts in adults are often symptomatic, cosmetically disabling, and difficult to treat. Killed Mycobacterium indicus pranii (previously known as Mycobacterium w, popularly known as Mw) vaccine has earlier been investigated in genital warts with encouraging results. Objective: To evaluate the efficacy and safety profile of intralesional injected killed Mw vaccine for the treatment of extensive extragenital cutaneous warts. Methods: In this study, a retrospective analysis of medical records was performed in patients with cutaneous warts treated with intralesional Mw vaccine. Only patients with more than 5 extra-genital warts, involving at least two body sites and which had not shown any signs of spontaneous regression over 6 months were treated with the vaccine. Results: Forty four patients were treated with intralesional Mw vaccine. The mean number of warts was 41.5 ± 25.7 with a disease duration of 3.1 ± 2.5 years. Complete clearance was achieved in 24 (54.5%) patients with a mean of 3.4 ± 1.1 intralesional injections. Cosmetically acceptable response to therapy (>75% clearance) was achieved in 37 (84.1%) patients. Wart response at distant sites was seen in 38 (86.3%) patients. Thirty-six patients (81.8%) experienced mild therapy-related side effects. Eighteen patients with complete response were followed up for 5.27 ± 1.7 months and none had recurrence of lesions. Conclusions: Killed Mw vaccine is safe and effective in the treatment of extensive cutaneous warts. Larger, preferably randomized controlled trials are needed to assess its efficacy vis a vis standard therapies for warts.INTRODUCTION
Cutaneous warts are the result of infection of the epidermis with human papilloma virus (HPV). Different HPV types are responsible for particular clinical varieties such as common, plane, intermediate, myrmecia, plantar, mosaic, ano-genital and oral warts. Precise epidemiological data for warts are not available but two large population-based studies from USA and Russia showed different prevalence figures of 0.84% and 12.9%, respectively. [1],[2] Warts affect children more than infants and adults and more commonly affect the extremities and face.
Although spontaneous resolution within 2 years has been reported in 65-78% of warts, [3] most patients and physicians opt to treat warts as they are cosmetically disfiguring and sometimes itchy and painful, especially on the soles. Ciconte et al. noted moderate-to-severe derangement in quality of life affecting social and leisure activities in 38.8% of patients with warts. [4] Poor prognostic indicators include warts in adults, long duration, involvement of palms and soles and large numbers; such cases are frequently resistant to therapy and are less likely to remit spontaneously.
Numerous therapeutic modalities are available for warts with variable success rates. These include topical therapies such as salicylic acid, tretinoin, podophyllotoxin, trichloroacetic acid, formaldehyde, 5-fluorouracil, photodynamic therapy and surgical/cytotoxic modalities such as cryotherapy, laser ablation, intralesional bleomycin, electrocautery, and surgical excision. [3],[5],[6],[7],[8] A recent Cochrane review of topical therapies for warts found that good quality data exists only for salicylic acid and cryotherapy and concluded that there was no clear evidence that any of the other treatments offered any advantage in terms of higher cure rates or fewer adverse effects. [9]
Treatment of multiple or refractory lesions is a challenge since destructive modalities can clear only the treated lesions and not the ones distant to the site of application. Immunotherapeutic modalities used for warts include contact sensitizers, imiquimod, intralesional interferons, and oral drugs such as levamisole, cimetidine, and zinc sulfate [10] but sound clinical data on the efficacy of these modalities are lacking. Immunomodulating agents have generated considerable interest as it is well known that cell mediated immunity is involved in the clearance of cutaneous warts. [11] Several intralesional antigens have been tried including MMR (measles, mumps, rubella) vaccine, skin test antigens (mumps, Candida, Trichophyton), BCG (Bacillus Calmette-Guerin) vaccine, and Candida antigen. [10],[12],[13],[14]
Killed Mycobacterium w (Mw) vaccine was developed in India and is derived from a nonpathogenic, rapidly growing, atypical Mycobacterium belonging to Runyon class IV. It has been exhaustively studied and approved as an immunotherapeutic adjunct to multidrug therapy of multibacillary leprosy in India. [15],[16] It is strongly antigenic and generates robust cytokine [interleukin-2 (IL-2), interferon-γ (IFN- γ)] and T-cell responses. [17] Its nomenclature was later changed to Mycobacterium indicus pranii but due to widespread use we have retained the name Mw in this paper. In an earlier study, we found encouraging results with intralesional Mw therapy for genital warts. [18] In this retrospective study we report the treatment response and adverse effects of intralesional injections of Mw for the treatment of long standing, extensive extragenital cutaneous warts.
METHODS
Study Design
We undertook a retrospective review of the medical records of all patients who were treated with intralesional Mw vaccine between August 2009 and July 2012 in the dermatology Outpatient Department at the All India Institute of Medical Sciences (AIIMS), New Delhi, India.
Patients
Patients who had more than five extragenital warts involving more than one body site or difficult to treat sites (periungual, palms, and soles), with no signs of spontaneous regression in the past 6 months were administered intralesional Mw vaccine. Informed consent for therapy was taken from all patients. Patients aged <18 and >70 years, pregnant and lactating women, suffering from chronic diseases such as diabetes/renal/hepatic illness, and immunocompromised patients (human immunodeficiency virus [HIV], other immunodeficiency disorders, patients taking immunosuppressive drugs, etc.) were excluded. All patients who received one or more intralesional injections of Mw were included in this analysis.
Treatment protocol of Mw vaccine
Mw vaccine (IMMUVAC; Cadila Pharmaceuticals, Ahmedabad, India.) is available in a multidose vial of 0.5 ml containing 500 million heat killed bacilli in a buffer solution. A sensitizing dose of the vaccine was administered intra-dermally at a dose of 0.1 mL, over deltoid area of each shoulder using an insulin syringe. After 2 weeks, the injected sites were examined for an immune response manifesting as persistent erythema or a nodule. In sensitized patients, 0.1 ml or less (4 units or less in the insulin syringe) of the vaccine was injected intra-epidermally or into the superficial dermis in 2 to 4 warts. Larger warts were preferred for injection. The injections were repeated at two weekly intervals till complete resolution or a total of 10 injections were administered, whichever was earlier.
Assessment of response
Response in both injected and un-injected warts was noted at each visit and a sequential clinical photographic record was maintained. Both local as well as patient-reported side effects were noted. The response was expressed as a percentage improvement from baseline based on physician assessment.
RESULTS
A total of 48 patients received Mw vaccine injections. Four patients either did not proceed with treatment after initial consent or took only the sensitizing dose and hence were not included in the analysis. Of the remaining 44 patients, 2 patients received 3 intralesional injections and were subsequently not contactable and the remaining 42 took at least 4 injections or followed up until complete resolution.
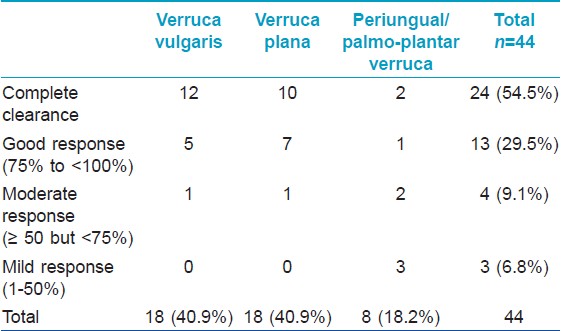
The results for 44 patients are summarized in [Table - 1]. Most of the patients were male (36/44, 81.8%). The mean duration of disease was 3.1 ± 2.5 years and the mean number of warts was 41.5 ± 25.7. The face was the most common site (26, 59.1%), followed by the upper (38.6%) and lower (25%) limbs. Only seven patients (15.9%) had warts on the trunk sparing the extremities. Multiple noncontiguous sites were involved in 19 patients (43.2%). Twenty patients (45.4%) had failed previous therapies which included electrosurgery (10 patients), salicylic acid/trichloroacetic acid (9 patients), homeopathic medications (4 patients), cryotherapy (2 patients), and oral levamisole (1 patient). There were 18 patients each with verruca vulgaris and plane warts while 8 patients had palmo-plantar/periungual warts [Table - 2].
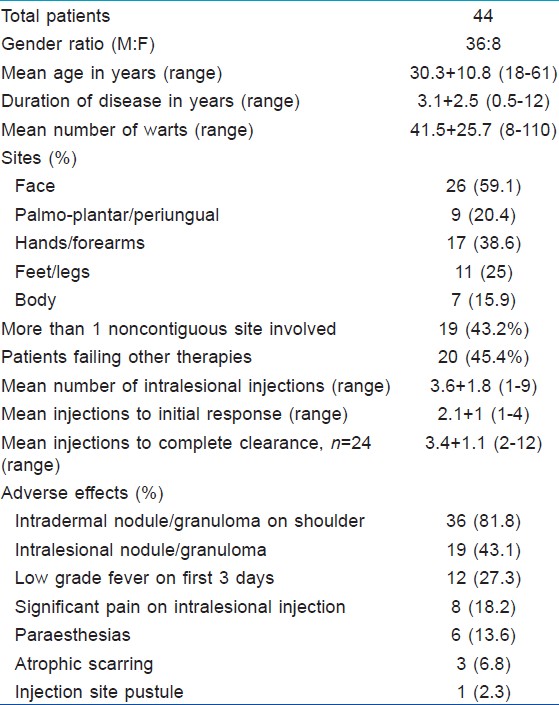
The mean number of intralesional injections taken before initiation of response was 2.1 ± 1 (range 1 to 4). Complete clearance was achieved in 24 (54.5%) patients and in these patients the mean number of intralesional injections required was 3.4 ± 1.1 (range 2 to 12).
The degree of clinical resolution attained in various types of warts is shown in [Table - 2]. Complete clearance was seen in 12 (66.7%) of 18 patients with verruca vulgaris, 10 (55.6%) of 18 patients with plane warts, and 2 (25%) of 8 patients with palmo-plantar or periungual warts. A cosmetically acceptable response to therapy (>75% clearance) was achieved in 37 (84.1%) patients (17 (94.4%) plane warts, 17 (94.4%) verruca vulgaris, and 3 (37.5%) palmo-plantar warts) Representative patients showing complete or nearly complete clinical remission are depicted in [Figure - 1] and [Figure - 2]. Thirty eight (86.3%) patients showed response in lesions distant to the site of injection (defined as 100% resolution of at least 1 wart at an anatomically distinct distant site). [12]
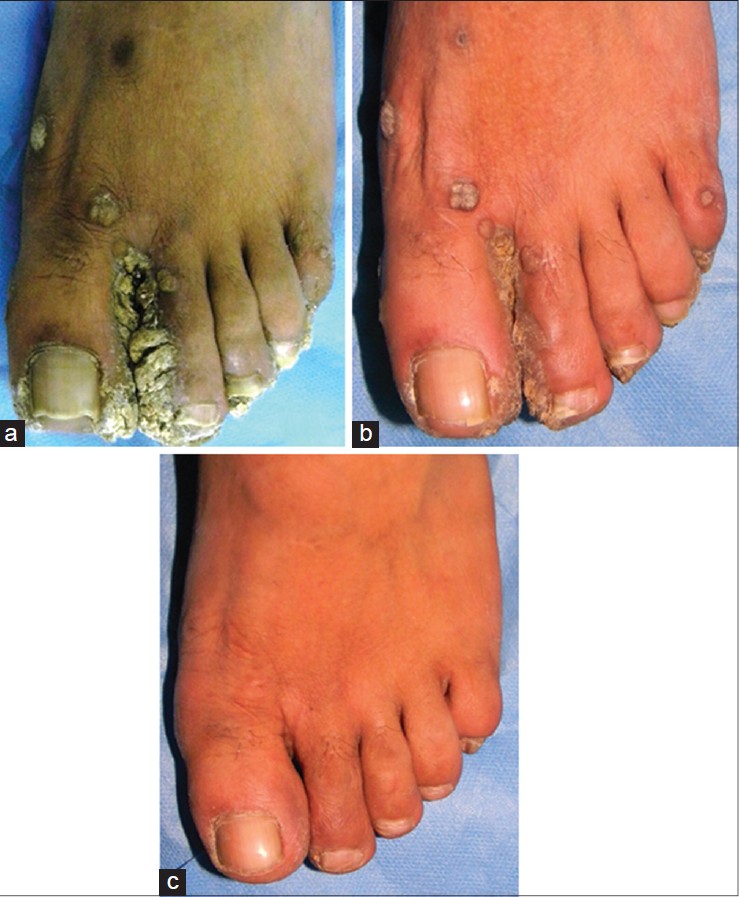 |
| Figure 1: Sequential clearance shown in composite photographs of left foot (patient 24). (a) Baseline – multiple hyperkeratotic warts in the fi rst web space, tips of toes, and few scattered over the dorsum of foot and toes. (b) After two injections – significant flattening of the web space warts. (c) After seven injections – complete clearance |
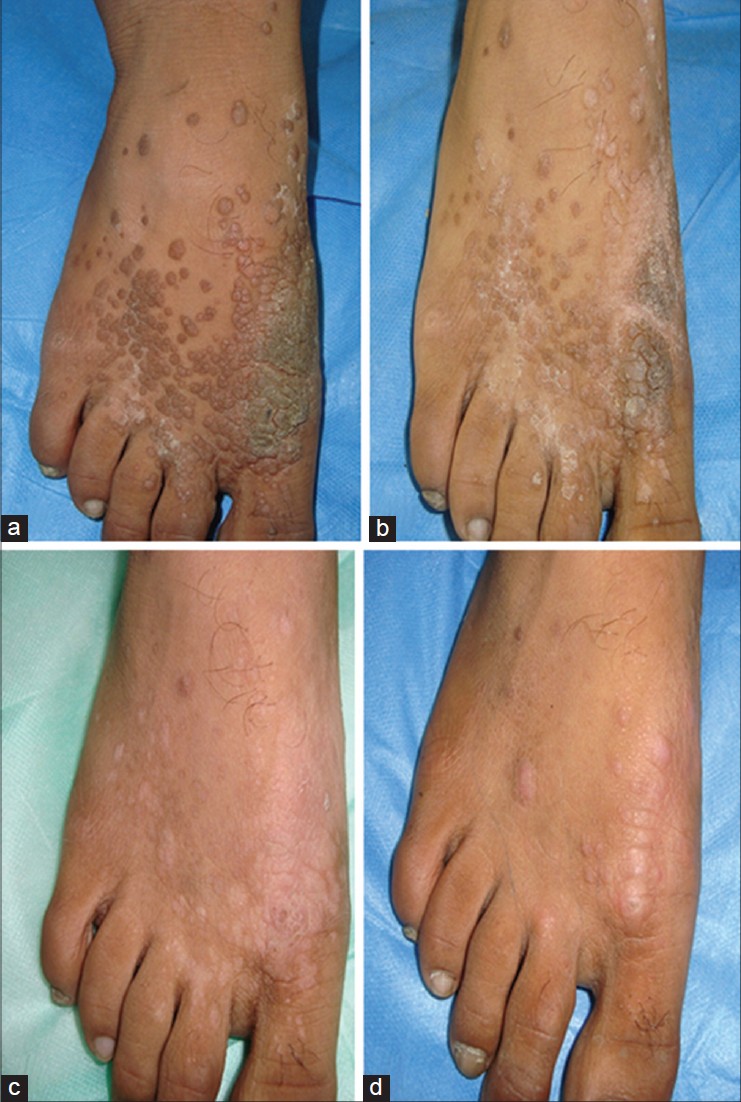 |
| Figure 2: Sequential clearance shown in composite photographs of right foot (patient 31). (a) Multiple verruca vulgaris (>50) localized to the dorsum of right foot at baseline, before intralesional injection of Mw vaccine. (b) After one injection – areas of clearing seen. (c) After three injections – significant improvement. (d) After five injections – more than 95% clearance. Injection sites are visible as dermal papules and nodules, thus indicating signifi cant resolution of un-injected distant warts as well |
Adverse effects were experienced by 36 (81.8%) patients. All 36 patients developed a nodule at the intradermal injection site on the shoulder. In two patients, the nodule ulcerated and healed with atrophic scarring in 4-5 weeks. In the remaining 34 patients, the nodules subsided spontaneously in 2-3 weeks without any residual changes. Nineteen (43.1%) patients developed nodules at the site of injected warts and 3 of these healed with depressed atrophic scarring in 2-4 weeks. In 4 patients, the nodules remained active (erythematous, mildly tender) until completion of the course of therapy. In the remaining patients, these nodules healed without any scar or pigmentation. Twelve (27.3%) patients experienced an episode of low-to-moderate grade fever, mostly on day 2 after the intradermal injection, which subsided without any antipyretic medications. Six (13.6%) patients experienced some vague altered sensations (paraesthesias) in the limbs distal to the injected warts which subsided spontaneously in 1 week. Pain was a prominent complaint in injected periungual/palmo-plantar warts (8 patients, 18.2%) and a sterile pustule at the injection site was noted in 1 patient.
Eight of 44 patients did not develop an intradermal nodule on the shoulder but had an erythematous papule or plaque at the intradermal injection site. The presence or absence of the nodule did not seem to affect or predict the clinical response to the injection.
There were no recurrences among 18 patients with complete clinical response during a follow up of 3-9 months (mean 5.27 ± 1.7 months).
DISCUSSION
Extragenital cutaneous warts in immunocompetent individuals are known to resolve on their own. A much cited study on an institutionalized population reported that warts subsided spontaneously within a 2-year period in 66.7% of patients. [19] Similarly, spontaneous regression has been noted in ′no treatment′/placebo groups of several placebo-controlled clinical trials over variable periods of time with cure rates ranging from 15% to 63%. [10],[12],[20],[21],[22] These findings may be explained by studies on spontaneously regressing ano-genital warts that showed a prominence of cell mediated immunity evidenced by increase in Th1 type cytokines (IL-2, tumor necrosis factor α, IFN α, β, and γ) and infiltration with CD4 T lymphocytes and macrophages in those warts. Keratinocytes are both the main source of the inflammatory cytokines as well as the main target of HPVs. [11],[23] Thus by manipulating the local immunological milieu, it may be possible to achieve a therapeutic response against both clinical and latent cutaneous HPV infections.
Resistant multiple cutaneous warts are a therapeutic challenge. Destructive therapies are ineffective for distant lesions and need multiple, often painful sessions with a risk of scarring. Various immunotherapeutic approaches including oral preparations like zinc sulfate, levamisole, cimetidine, etretinate, and topical/injectable preparations including contact sensitizers (dinitrochlorobenzene, diphencyprone, squaric acid dibutyl ester), interferons (α-2a, β, and γ) and imiquimod have been used but limited data are available to support their use. [5]
Another approach, which is being increasingly investigated for the treatment of multiple warts is intralesional injections of various antigens for their nonspecific vaccine-like immunostimulating effect against HPV, especially for inducing clearance of lesions distant to the site of application. In a prospective clinical trial comparing cryotherapy with intralesional mumps and candida skin test antigens for warts, complete cure was noted in 43% in the cryotherapy group and 52% in the immunotherapy group. Twenty-nine patients (74%) had distant wart resolution. Six (11.1%) patients in the immunotherapy group had a flu-like illness lasting less than 24 h and injection site pain and pruritus. [24] Clifton et al. found these antigens to be safe and effective in pediatric recalcitrant warts as well with a cure rate of 47%, while 34% patients experienced resolution of distant warts. [25] Horn et al. compared the efficacy of skin test antigens (mumps, candida, and trichophytin), IFN α-2b and placebo injection in a randomized controlled trial. More than 75% clinical resolution was seen in 54% of the patients injected with antigen alone and 68% of patients injected with antigen plus interferon. Side effects seen included fever, myalgias and injection site erythema and edema. [12] Intradermal BCG vaccine has also been evaluated for warts and found to produce complete clinical remission in 39.7% of patients. [13] Considering its immunotherapeutic potential, Salem et al. evaluated topical BCG vaccine for warts in children and noted a complete response in 65% patients with common warts and 45% with plane warts. [26] More recently, MMR vaccine was evaluated as a treatment option for cutaneous warts in a randomized placebo-controlled trial. A complete response was achieved in 81.4% of the patients in the MMR group as compared with 27.5% of patients in the placebo group. Injection site pain and flu-like symptoms were the only side effects noted in this study. [10]
Mw vaccine appears to be an efficacious and safe treatment modality for extensive, long standing/resistant cutaneous warts. We achieved complete remission in 54.5% patients after a mean of 3.4 + 1.1 injections and cosmetically significant response in 84.1% patients. Therapy was relatively well tolerated. In an earlier open label pilot study of intralesional Mw vaccine on patients with external ano-genital warts, 8 of 9 patients (88.9%) achieved complete clinical clearance after a mean treatment duration of 5.9 weeks (similar to 6.75 weeks in the current study). [18] In another recent prospective open label study, 83% of cutaneous warts treated with weekly intralesional Mw vaccine resolved completely. Mean time to complete response was 9.7 weeks (versus 6.75 weeks in the current study) and distant wart response was noted in 70% (versus 86.3% in current study). [27]
The exact mechanism of action of Mw vaccine against viral warts is not known. Experimental evidence suggests that it leads to a strong Th1 cytokine response, especially increased production of IFN-γ, increased T cell and macrophage activation and also increasing CD8+ T cell response. Levels of interleukins 4 and 5 (Th2 cytokines which cause deficient cellular immune response) were not altered by the vaccine and immune responses were improved when the vaccine was administered with a Ribi adjuvant. This strong pro-Th1 effect may be responsible for its clinical effectiveness for warts. Because of these favorable immunomodulatory effects and the fact that it shares antigens with Mycobacterium tuberculosis, it has also been successfully used as an immunotherapeutic agent for pulmonary tuberculosis both in experimental and clinical settings. [17],[28],[29] Imiquimod has shown good efficacy in the treatment of ano-genital warts secondary to its prominent stimulation of Th1 cytokines like IFN (α, β, γ), TNF α, and IL 2. [30] These similarities in the immunomodulating properties of imiquimod and Mw vaccine prompted us to use the latter for warts.
The patho-mechanism of other intralesional immunotherapies is also unknown but proposed mechanisms include induction of a strong non-specific inflammatory response against HPV infected cells, trauma itself or the bystander effect that may cause wart clearance in previously sensitized individuals, and, finally, antigen injection may be associated with proliferation of peripheral blood mononuclear cells that promote Th1 cytokine responses, which further activate cytotoxic T cells and natural killer cells to eradicate HPV-infected cells. [12],[18],[24]
Most reports on the use of intralesional therapy for warts are open label studies and there is a dearth of randomized controlled trials investigating their role. Immunotherapy is inexpensive especially for extensive warts and hence can be of special value in developing countries. [31] Although our study is limited by its retrospective nature, the results are encouraging. Well designed, large randomized placebo-controlled clinical trials should be performed before Mw vaccine can be definitively recommended for treatment of extensive cutaneous warts in clinical practice.
| 1. |
Johnson MT, Roberts J. Skin conditions and related need for medical care among persons 1-74 years. United States, 1971-1974. Vital Health Stat-11 1978;1-72.
[Google Scholar]
|
| 2. |
Beliaeva TL. The population incidence of warts. Vestn Dermatol Venerol 1990;55-8. [Russian].
[Google Scholar]
|
| 3. |
Sterling JC, Handfield-Jones S, Hudson PM; British Association of Dermatologists. Guidelines for the management of cutaneous warts. Br J Dermatol 2001;144:4-11.
[Google Scholar]
|
| 4. |
Ciconte A, Campbell J, Tabrizi S, Garland S, Marks R. Warts are not merely blemishes on the skin: A study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol 2003;44:169-73.
[Google Scholar]
|
| 5. |
Dall'Oglio F, D'Amico V, Nasca MR, Micali G. Treatment of cutaneous warts: An evidence based review. Am J Clin Dermatol 2012;13:73-96.
[Google Scholar]
|
| 6. |
Gibbs S, Harvey I, Sterling J, Stark R. Local treatments for cutaneous warts: Systematic review. BMJ 2002;325:461.
[Google Scholar]
|
| 7. |
Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: A meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol 2011;165:233-46.
[Google Scholar]
|
| 8. |
Bacelieri R, Johnson SM. Cutaneous warts: An evidence based approach to therapy. Am Fam Physician 2005;72:647-52.
[Google Scholar]
|
| 9. |
Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev 2012:9:CD001781.
[Google Scholar]
|
| 10. |
Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol 2010;24:1166-70.
[Google Scholar]
|
| 11. |
Goncalves MA, Donadi EA. Immune cellular response to HPV: Current concepts. Braz J Infect Dis 2004;8:1-9.
[Google Scholar]
|
| 12. |
Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with Mumps, Candida and Trichophytin skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol 2005;141:589-94.
[Google Scholar]
|
| 13. |
Sharquie KE, Al-Rawi JR, Al-Nuaimy AA, Radhy SH. Bacille Calmette-Guerin immunotherapy of viral warts. Saudi Med J 2008;29:589-93.
[Google Scholar]
|
| 14. |
Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O'Bryan K, et al. Phase I clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol 2010;146:1431-3.
et al. Phase I clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol 2010;146:1431-3.'>[Google Scholar]
|
| 15. |
Talwar GP. An immunotherapeutic vaccine for multibacillary leprosy. Int Rev Immunol 1999;18:229-49.
[Google Scholar]
|
| 16. |
Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, Parida SK, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr Rev 2005;76:127-43.
[Google Scholar]
|
| 17. |
Singh IG, Mukherjee R, Talwar GP, Kaufmann SH. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect Immun 1992;60:257-63.
[Google Scholar]
|
| 18. |
Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol 2008;22:1089-93.
[Google Scholar]
|
| 19. |
Massing AM, Epstein WL. Natural history of warts. A two-year study. Arch Dermatol 1963;87:306-10.
[Google Scholar]
|
| 20. |
Gustafsson L, Leijonhufvud I, Aronsson A, Mossberg AK, Svanborg C. Treatment of skin papillomas with topical alpha-lactalbumin-oleic acid. N Engl J Med 2004;350:2663-72.
[Google Scholar]
|
| 21. |
Varnavides CK, Henderson CA, Cunliffe WJ. Intralesional interferon: Ineffective in common viral warts. J Dermatol Treat 1997;8:169-72.
[Google Scholar]
|
| 22. |
Bruggink SC, Gussekloo J, Berger MY, Zaaijer K, Assendelft WJ, de Waal MW, et al. Cryotherapy with liquid nitrogen versus topical salicylic acid application for cutaneous warts in primary care: Randomized controlled trial. CMAJ 2010;182:1624-30.
[Google Scholar]
|
| 23. |
Grassegger A, Rollinger-Holzinger I, Zelger BW, Heim K, Zwierzina H, Fritsch PO, et al. Spontaneous or interferon-γ-induced T-cell infiltration, HLA-DR and ICAM-1 expression in genitoanal warts are associated with TH1 or mixed TH1/TH2 cytokine mRNA expression profiles. Arch Dermatol Res 1997;289:243-50.
[Google Scholar]
|
| 24. |
Johnson SM, Roberson PK, Horn TD. Intra-lesional injection of mumps or candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol 2001;137:451-5.
[Google Scholar]
|
| 25. |
Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intra-lesional mumps or candida antigens. Pediatr Dermatol 2003;20:268-71.
[Google Scholar]
|
| 26. |
Salem A, Nofal A, Hosny D. Treatment of common and plane warts in children with topical viable Bacillus Calmette-Guerin. Pediatr Dermatol 2013;30:60-3.
[Google Scholar]
|
| 27. |
Meena J, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol 2013;149:237-9.
[Google Scholar]
|
| 28. |
Faujdar J, Gupta P, Natarajan M, Das R, Chauhan DS, Katoch VM, et al. Mycobacterium indicus pranii as stand-alone or adjunct immunotherapeutic in treatment of experimental animal tuberculosis. Indian J Med Res 2011;134:696-703.
[Google Scholar]
|
| 29. |
Patel N, Trapathi SB. Improved cure rates in pulmonary tuberculosis category II (retreatment) with Mycobacterium w. J Indian Med Assoc 2003;10:680,682.
[Google Scholar]
|
| 30. |
Tyring SK, Arany I, Stanley MA, Tomai MA, Miller RL, Smith MH, et al. A randomized, controlled, molecular study of condylomata acuminata clearance during treatment with imiquimod. J Infect Dis 1998;178:551-5.
[Google Scholar]
|
| 31. |
Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol 2011;77:261-3.
[Google Scholar]
|
Fulltext Views
5,354
PDF downloads
2,740





