Translate this page into:
Intralesional measles, mumps, and rubella vaccine versus vitamin D for treatment of warts: A randomised clinical trial
Corresponding author: Dr. Amany Awad, Department of Dermatology, Andrology and Sexually Transmitted Diseases, Faculty of Medicine, Mansoura University, Mansoura, Egypt. ama.awad2007@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sallam M, Awad A, Hamdy S, State A. Intralesional measles, mumps, and rubella vaccine versus vitamin D for treatment of warts: A randomised clinical trial. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1669_2024
Abstract
Background
Warts are prevalent distressing skin growths caused by the human papillomavirus (HPV). These growths are commonly addressed using methods that destroy the tissue, including chemical cautery, electrocautery, or cryotherapy. These methods have many side effects in contrast to intralesional immunotherapy.
Objectives
This study was conducted to assess the effectiveness, safety, and tolerability of utilising the intralesional measles, mumps, and rubella (MMR) vaccine compared to vitamin D in warts treatment.
Methods
This randomised clinical trial enrolled 112 participants presenting with multiple warts. The participants were sub-divided into two groups through a random allocation process. Group Ⅰ (n=56) was administered 0.3 mL intralesional MMR vaccine, whereas group Ⅱ (n=56) was administered 0.3 mL intralesional vitamin D3 (equivalent to 15000 IU cholecalciferol). The injection was administered every two weeks into the most noticeable wart, requiring no more than five sessions until improvement. A follow-up period of six months was conducted after the final treatment session.
Results
A significantly higher percentage of complete response was noticed in the MMR group (80.4%) as compared with the vitamin D group (66.1%). Both groups had an average of four sessions, showing no significant difference. Regarding adverse effects, the MMR group demonstrated a significantly greater incidence of mild pain (96.4%) and injection site itching (12.5%) compared with the vitamin D group. After 6 months of follow-up, no significant difference was noticed in recurrence rates in both groups (3 cases; 5.4% in the vitamin D vs. 2 cases; 3.6% in the MMR group).
Conclusion
Intralesional MMR demonstrates greater efficacy than vitamin D in treating warts but with a higher incidence of tolerable side effects
Keywords
Intralesional
MMR
vitamin D and immunotherapy
warts
Introduction
Warts, induced by the various human papillomavirus (HPV) strains, are widespread and upsetting skin growths infecting the outer skin layers and/or mucous membranes.1 Although the diagnosis can often be made clinically, dermoscopy plays a crucial role in confirming the diagnosis and following up the response to treatment.2
Dermatologists should consider the recurrent and benign nature of warts while establishing the treatment plan.3 Warts were typically treated with destructive methods, such as chemical cautery, electrocautery, or cryotherapy. These methods may cause many side effects, like inflammation, scarring, hypo-, or hyper-pigmentation. Additionally, treatment response is expected in the target wart only. In contrast, intralesional immunotherapy is linked to a lower incidence of adverse effects and works by activating the immune system to identify the virus, resulting in treatment response in all warts.2
Various antigens, such as BCG, PPD, MMR, Candida antigen, and the more recently utilised HBV vaccine, have been injected as immunotherapy for the treatment of warts.1 The MMR vaccine functions as an immunotherapy by eliciting a Th1 immune response, increasing IL-2, IL-4, IL-5, TNF α, and IFN-γ, and inducing delayed hypersensitivity targeting both MMR viral antigens and HPV.4
Nevertheless, vitamin D3 acts by regulating cytokine production and epidermal proliferation, which contributes to its effectiveness over other immunotherapeutic approaches. It suppresses IL-1 α and IL-6 while stimulating toll-like receptors (TLRs) of human macrophages, promoting the production of antimicrobial peptides in both treated and remote warts.5 Despite numerous reports regarding the efficacy of intralesional immunotherapy in wart treatment, it remains unapproved by the FDA. So, additional research is needed to compare different antigens and gain a clearer understanding of their relative efficacy.3
The main aim of this randomised clinical trial was to assess the effectiveness of the intralesional measles, mumps, and rubella (MMR) vaccine over vitamin D in treating warts. Moreover, the secondary objectives were to assess their safety and tolerability. Dermoscopy was applied as a tool to confirm the clinical diagnosis of complete improvement.
Methods
The research received approval from the ethics committee of the Faculty of Medicine, Mansoura university hospital, number 2617 dated may 2024. This randomised clinical trial enrolled 112 patients with multiple common, plantar and,/or plane warts, clinically diagnosed and verified through dermoscopic evaluation. All participants were selected from the Dermatology Outpatient Clinic at Mansoura university hospital from April 2022 to October 2023.
Exclusion criteria in the current study were: (i) patients aged < 4 years, (ii) prior diagnosis of asthma, allergic skin conditions, widespread dermatitis, hypersensitivity to vitamin D, or MMR, (iii) pregnant or breastfeeding women, (iv) patients with compromised immune system—(either absolute or relative), and (v) individuals with chronic illnesses, including kidney failure, liver dysfunction, hepatitis, or heart-related conditions. Moreover, participants who had undergone any treatment for warts within the three months preceding the study was excluded.
Prior to their participation in the study, written informed consents were secured from all patients or their caregivers. The CONSORT flow diagram of participants has been illustrated in Figure 1.

- consort flow chart showing the included, excluded and studied groups.
Each participant underwent a thorough medical history review, along with a comprehensive general and dermatological evaluation to determine the quantity, dimensions, and location of warts, while ruling out any additional skin disorders.
Digital images were captured before injection and during each follow-up visit for each patient using Sony Cyber-shot DSC-W620.
DermLite III device from 3 Gen was utilised for Dermoscopy to confirm wart diagnoses prior to treatment and during every session throughout the study to assess the level of improvement following treatment. Two dermatologists, blinded to the study details, assessed the dermoscopic photomicrographs to assess the clinical and dermoscopic improvement.
Sample size calculation
The study’s sample size was determined to be 56 participants per group using the G*Power 3.1.9.7 (2020) software, based on a 5% significance level and 80% statistical power. It was calculated using the proportion of complete response among MMR vaccine group was (80%) while represent (56%) among vitamin D group based on Shaldoum, et al.4
Participants were allocated into two groups of 56 individuals each, utilising computer-generated random numbers placed in opaque, sealed envelopes. A topical anaesthetic cream was applied to the site of injection 30 minutes prior to the procedure.
Patients in Group Ⅰ were administered intralesional MMR (0.5 mL freeze-dried vials from VACSERA, Egypt). The solution was prepared by diluting it with sterile distilled water (0.5 mL), and each individual received a 0.3 mL MMR direct injection into the largest wart without prior sensitisation. Patients in Group Ⅱ were given 0.3 mL intralesional vitamin D3 (MPCI.CA, Egypt) (2.5 mL corresponding to 15.000 IU cholecalciferol) into the largest wart.
Insulin syringes were used for the injections. Each patient received repeat treatments into the same wart every 2 weeks until the lesions cleared or until five sessions were completed.
Assessment of the response
The treatment outcomes, encompassing both the directly treated and untreated distant warts, were evaluated and classified into three categories: complete response (wart disappearance and the skin returned to its normal state), partial response (50%–99% decrease in wart size or quantity), and poor response (0%–50% decrease in wart size or quantity).
The process involved assessing the number and dimensions of both treated and untreated warts, which were recorded through digital photographs taken under consistent camera and lighting conditions. Additionally, dermoscopic analysis was used to identify the reappearance of skin markings and the eradication of thrombosed blood vessels.
Safety assessment
At every treatment session, local reactions like pain, redness, swelling, and itching, as well as systemic symptoms resembling influenza occurring within 12 hours post-injection, were documented.
Follow-up
The clinical response was monitored biweekly throughout the treatment phase and continued bimonthly for six months following the final session.
The clinical response was recorded and assessed 2 weeks after the final treatment session. However, recurrence was tracked over a six-month period after the final session.
Statistical analysis and data interpretation
Data were analysed utilising the Statistical Package of Social Science (SPSS) program (version 24). Testing for data normality was achieved utilising the one-sample Kolmogorov-Smirnov test. Qualitative data were expressed utilising numbers and percentages. For data following a normal distribution, continuous variables were expressed as mean ± standard deviation (SD), while for non-parametric data, they were expressed by the median along with the range (min-max). The following tests were applied:
Chi-square test: To compare qualitative variables. Independent t-test: To compare two quantitative variables (parametric). Fisher exact test: To compare qualitative variables if the expected frequency was below 5.
For all statistical analyses, the significance threshold is fixed at the 5% level. Results were deemed significant if p ≤ 0.05.
Results
The included patients were divided into two groups randomly, with no significant differences in age, gender, or the initial clinical characteristics of the studied warts [Table 1].
| Patient’s characteristics |
MMR group (n=56) |
Vitamin D group (n=56) | P-value |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 26.23±12.87 | 32.7±13.6 | 0.088 |
| Sex | |||
| Male | 17 (30.4%) | 14 (25.0%) | 0.526 |
| Female | 39 (69.6%) | 42 (75.0%) | |
| Duration of warts (month) | |||
| Mean ± SD | 17.38 ± 9.20 | 21.18 ± 9.80 | 0.070 |
| Size of warts (mm) | |||
| Mean ± SD | 5.64 ± 3.227 | 5.39 ± 3.489 | 0.740 |
| Previous treatment | |||
| Yes | 37 (66.1%) | 30 (53.6%) | 0.177 |
| No | 19 (33.9%) | 26 (46.4%) | |
| Type of warts | |||
| Common | 19 (33.9%) | 22 (39.3%) | 0.817 |
| Planter | 26 (46.5%) | 23 (41.1%) | |
| Plane | 11 (19.6%) | 11 (19.6%) |
SD: Standard deviation
Table 2 demonstrates a significantly greater percentage of complete response in MMR (80.4%) relative to the vitamin D group (66.1%). Partial responders were also higher in the MMR (14.3%) than in the vitamin D group (7.1%), while the percentage of poor responders was greater in the vitamin D group (26.8%) than in the MMR group (5.4%) with no significant differences in the average number of required injections (4 sessions) in both groups [Figures 2-5].
| Response |
MMR group (n=56) |
Vitamin D group (n=56) | Test of significance | P-value |
|---|---|---|---|---|
| Complete response | 45 (80.4%) | 37 (66.1%) | χ2=10.11 | 0.08* |
| Partial response | 8 (14.3%) | 4 (7.1%) | ||
| No response | 3 (5.4%) | 15 (26.8%) | ||
| Number of injections | ||||
| Mean ± SD | 4 ± 1.19 | 4.11 ± 1.16 | t= 0.544 | 0.588 |
SD: Standard deviation, *statistically significant
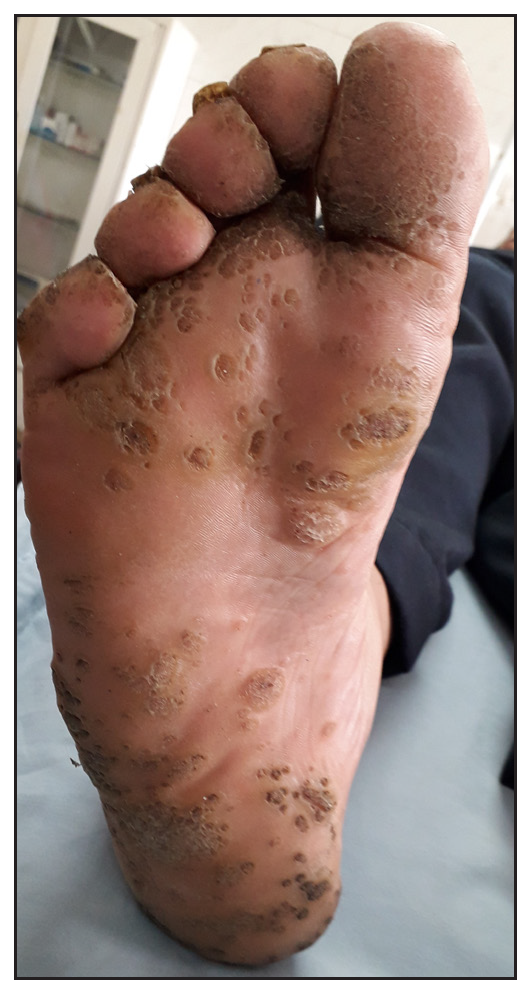
- Male patient aged 25 y in the MMR group with multiple plantar warts, (before treatment).
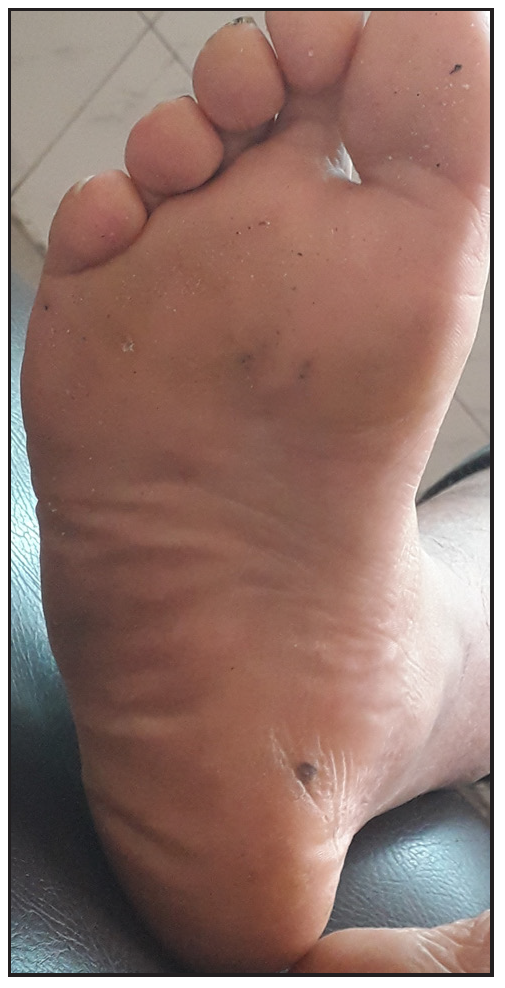
- After five sessions of IL MMR with excellent response, (polarised, 20x).
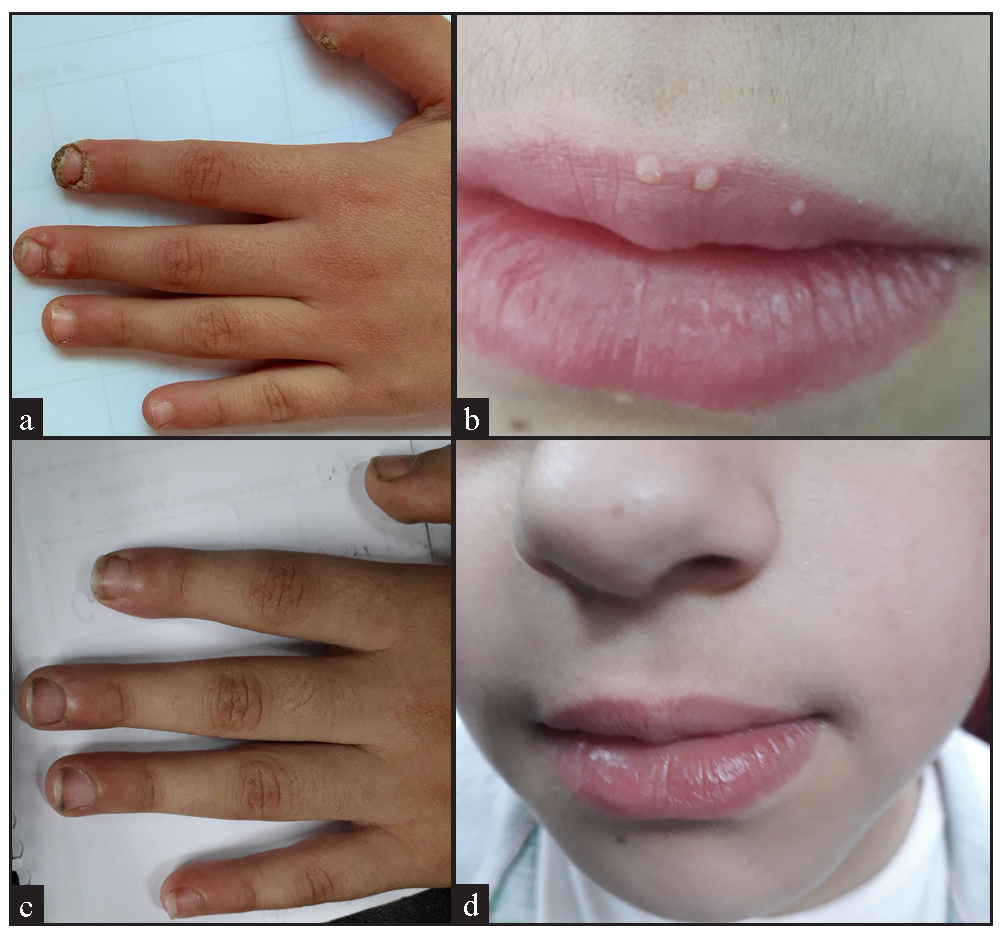
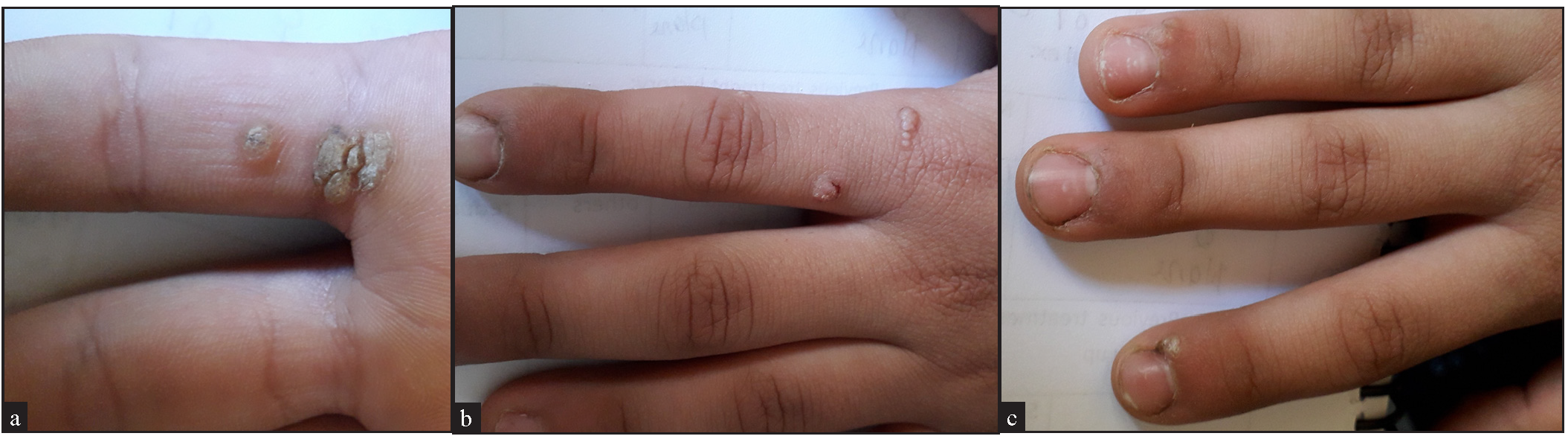
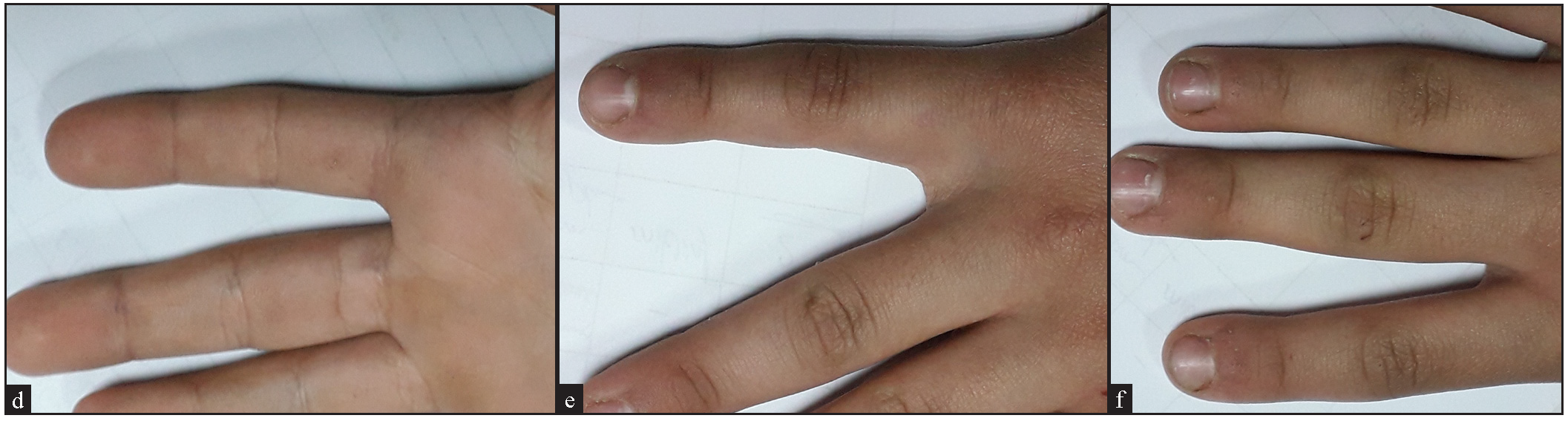
- (a) A 10-year old girl in the MMR group with multiple common and periungual wartswarts, (before treatment), (b) Female patient aged 10 years in the vitamin MMR group with multiple common and periungual warts, (before treatment), (c) After three sessions of IL MMR with excellent response, (d) After three sessions of IL MMR with excellent response.
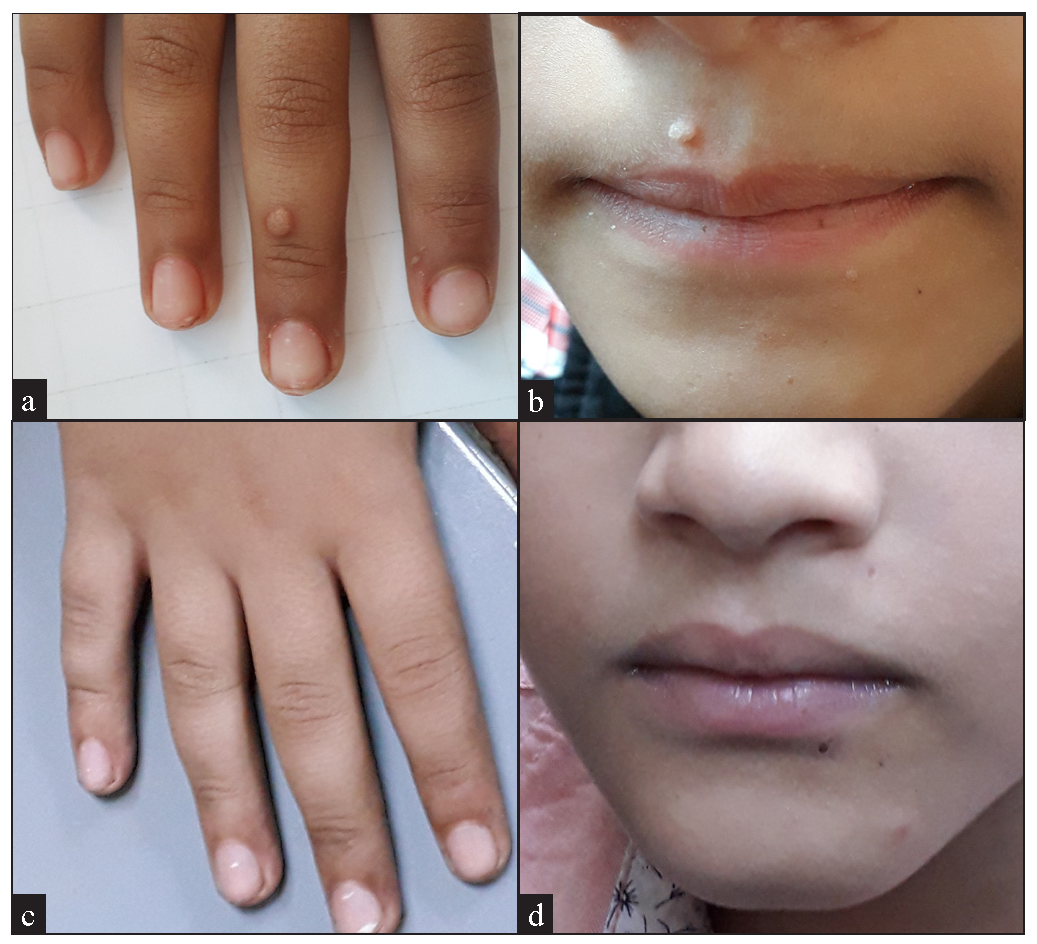
- (a) A 6-year old boy with multiple common warts, (before treatment), (b) Male patient aged 6 years in the vitamin D group with multiple common warts, (before treatment), (c) A 6-year old boy with multiple common warts, (after treatment), (d) After 3 sessions of IL vitamin D with excellent response.
- A 9-years old boy in the vitamin D group with multiple common and periungual warts, (before treatment).
- After 4 sessions of IL vitamin D with excellent response
Regarding the adverse effects, the MMR group showed a significantly higher percentage of mild pain (96.4%) and injection site itching (12.5%) than the vitamin D group. Vitamin D group patients reported no injection site reactions. Severe pain, erythema, oedema, and symptoms resembling influenza were recorded in the MMR group more than in the Vitamin D group, though these differences were not statistically significant [Table 3].
| Side effects |
MMR group (n=56) |
Vitamin D group (n=56) | Test of significance | P-value |
|---|---|---|---|---|
| Mild-tolerable pain | 54 (96.4%) | 11 (19.6%) | χ2=67.8 | ≤0.001* |
| Severe pain | 2 (3.5%) | 0 (0%) | FET | 0.495 |
| Oedema | 1 (1.7%) | 0 (0%) | FET | 1.0 |
| Erythema | 6 (10.7%) | 1 (1.8%) | FET | 0.113 |
| Itching | 7 (12.5%) | 0 (0%) | FET | 0.013* |
| Flu-like symptoms | 3 (5.3%) | 0 (0%) | FET | 0.243 |
According to our results, in the MMR group, complete response occurred significantly with smaller size warts (mean = 3.8 mm) compared to larger warts [Table 4]. Conversely, in the vitamin D group, complete response was significantly more common in patients with plantar warts compared to other subgroups [Table 4].
| Patient’s characteristics | MMR group (n=56) | Test of significance | P-value | |
|---|---|---|---|---|
| Complete response (n=45) |
Partial response & no response (n=11) |
|||
| Size of warts (mm) | ||||
| Mean ± SD | 3.82 ± 1.3 | 5.7 ± 2.2 | t=3.71 | 0.005* |
| Type | ||||
| Common | 15 (33.3%) | 4 (36.3%) | χ2=0.728 | 0.695 |
| Planter | 22 (48.9%) | 4 (36.3%) | ||
| Plane | 8 (17.8%) | 3 (27.4%) | ||
| Patient’s characteristics | Vitamin D group (n=56) | Test of significance | P-value | |
| Complete response (n=37) |
Partial response & no response (n=19) |
|||
| Size of warts (mm) | ||||
| Mean ± SD | 4.89 ± 2.17 | 5.10 ± 3.2 | t= 0.29 | 0.772 |
| Type | ||||
| Common | 10 (27.1%) | 12 (63.2%) | χ2=6.91 | 0.032* |
| Planter | 18 (48.6%) | 5 (26.3%) | ||
| Plane | 9 (24.3%) | 2 (10.5%) | ||
SD: Standard deviation, *Statistically significant
After six months of follow-up, the recurrence rates revealed no significant difference between the two groups, with two cases (3.6%) in the MMR group versus three cases (5.4%) in the vitamin D group developing recurrence.
Discussion
In this study, 80.4% of participants from the MMR group experienced a complete response, while partial and no responses were observed in 14.3% and 54%, respectively. These results are consistent with Nofal and Nofal,6 Shaldoum et al.,4 and Mohammed et al.7 However, Awal and Kaur,8 Nofal et al.,9 Grawal et al.,10 Rezk et al.,11 and Mohamed and ElGhareeb12 reported lower response rates [Table 5]. This discrepancy may be owed to differences in sample sizes, ethnic backgrounds, doses, or intervals between sessions.
| MMR group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Current study | Awal and Kaur(8) | Nofal et al(9) | grawal et al(10) | Rezk et al(11) | Nofal and Nofal(6) | Shaldoum et al(4) | Yasser et al(7) | Mohamed and ElGhareeb(12) | |
| Complete response | 80.4% | 68% | 63% | 60% | 70% | 81.4% | 80% | 80% | 72.5% |
| Vitamin D | |||||||||
| Current study | Aktaş et al(15) | Kavya et al(16) | Shaldoum et al(4) | Raghukumar et al(17) | Abou-Taleb et al(19) | Kumar Singh et al(13) | Al-Sabak et al(18) | Mohta et al(14) | |
| Complete response | 66.1% | 80% | 78.5% | 66.7% | 90% | 43.5% | 72.5% | 81.9% | 77.4% |
It is supposed that the MMR vaccine, having three different antigens (measles, mumps, and rubella), may elicit an enhanced immune response to HPV by stimulating the release of multiple cytokines, including IL-2, IL-4, IL-5, and tumour necrosis factor-α.8)
In the present investigation, the administration of intralesional vitamin D injections led to a complete response in 66.1% of participants, while 7.1% exhibited a partial response, and 26.8% showed no improvement. These outcomes resemble those recorded by Shaldoum et al.,4 Rezk et al.13 and Mohta et al.14 However, Aktaş et al.,15 Kavya et al.,16 Raghukumar et al.,17 and Al-Sabak et al.18 reported higher response rates in contrast to Abou-Taleb et al.19 who reported lower response rates [Table 5]. The variation between these studies may be explained by using different concentrations, doses, and intervals. Additionally, to ensure accuracy and to minimise the potential for error associated with clinical examination alone, we verified the complete resolution of warts through dermoscopic evaluation.
The response of warts to intralesional MMR (80.4%) was significantly higher than to vitamin D (66.1%), aligning our study with prior research.4,5,14,20 The superior response in the MMR group can be explained by the synergistic effect of its three antigens, which generate a stronger immunogenic response.
In this study, both groups demonstrated a similar number of sessions needed to achieve a complete response (4). This finding indicates that injections should be discontinued if no improvement is observed by the fourth session. These results are consistent with those of previous studies.5,14 However, Shaldoum et al.4 reported significantly fewer sessions with vitamin D (2.9) compared to the MMR group (5.4), and this fast cure could be attributed to the injection of every wart with vitamin D in their study. Also, Naresh Babu et al.21 reported a slightly lower number of sessions in the vitamin D group than in the MMR group, and this might be related to the administration of elevated vitamin D doses.
Regarding adverse effects, the MMR group showed a notably increased proportion of mild pain (96.4%), and injection site itching (12.5%) compared to the vitamin D group. Vitamin D group patients reported no injection site reactions. Severe pain, erythema, oedema, and symptoms resembling influenza were recorded in MMR more than in the vitamin D group, with no significant results. These results are similar to those of Shaldoum et al.4 and Jartarkar et al.,5 but contrasting with Mohta et al.14 and Jain et al.22 who reported injection site itching exclusively in the Vitamin D group.
According to our results, complete response was significantly associated with smaller-sized warts (mean=3.8) in the MMR group, compared to those with partial or no response cases. In contrast, complete response was significantly related to plantar warts in the vitamin D group. Shaldoum et al.4 reported no correlation between the clinical characteristics of warts and the clinical response. Joshi et al.23 reported a significant inverse correlation between the length of time for which warts persisted and the response rate in the two groups.
Upon follow-up of patients for 6 months after cure, recurrence rates revealed no significant differences between the two groups, with three cases (5.4%) in the vitamin D group vs. two cases (3.6%) in the MMR group, which agrees with the results of Jain et al.22 In contrast, Mohta et al.14 reported no recurrences in the MMR group versus two (6.5%) patients in the vitamin D group, who had initially shown partial improvement. Shaldoum et al.4, Jartarkar et al.,5 and Babu et al.21 reported no recurrence in either group. Joshi et al.23 reported higher recurrence rates in the Vitamin D (14%) versus the MMR group (16%).
Limitations
This is a clinical study, and we did not assess viral types nor measured the serum levels of any involved cytokine pre and post treatment. Further studies are necessitated to assess the effect of these injections on the serum level of different cytokines.
Conclusion
Both intralesional MMR and vitamin D are efficacious, safe, and cost effective therapeutic modalities for warts with low recurrence rates. Therefore, immunotherapy may be used for multiple, disseminated, and recalcitrant warts.
Ethical approval
The research/study was approved by the Institutional Review Board at Mansoura University, number 2617, dated 11 May 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Intralesional immunotherapy for the treatment of warts: A mini review. Zagazig Univ Med J. 2024;30:313-6.
- [Google Scholar]
- Intralesional purified protein derivative versus zinc sulfate 2% in the treatment of pediatric warts: Clinical and dermoscopic evaluation. J Cosmet Dermatol. 2022;21:4637-4645.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of intralesional therapies for cutaneous warts. JID Innov. 2024;4:100264.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative clinical study of the efficacy of intralesional MMR vaccine vs intralesional vitamin D injection in treatment of warts. J Cosmet Dermatol. 2020;19:2033-2040.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of therapeutic efficacy of intralesional measles, mumps, and rubella vaccine and intralesional vitamin D3 in the treatment of recurrent warts. J Dermatol Dermatol. 2021;25:14.
- [CrossRef] [Google Scholar]
- Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166-70.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of intralesional tuberculin protein purified derivative (PPD) and intralesional measles, mumps, rubella (MMR) vaccine for multiple resistant warts. J Cosmet Dermatol. 2021;20:868-74.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic outcome of intralesional immunotherapy in cutaneous warts using the mumps, measles, and rubella vaccine: A randomized, placebo-controlled trial. J Clin Aesthet Dermatol. 2018;11:15-20.
- [PubMed] [PubMed Central] [Google Scholar]
- Treatment of recalcitrant warts with intralesional measles, mumps, and rubella vaccine: A promising approach. Int J Dermatol. 2015;54:667-71.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized double blind controlled study comparing the efficacy of intralesional MMR vaccine with normal saline in the treatment of cutaneous warts. Indian Dermatol Online J. 2018;9:389-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect of intralesional injection of MMR in treatment of viral warts. Benha J Appl Sci. 2021;6:223-6.
- [CrossRef] [Google Scholar]
- Comparative study of autoimplantation therapy and intralesional injection of MMR vaccine in warts treatment. Dermatol Ther. 2019;32:e13135.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts. Int J Res Dermatol. ;4:197-201.
- [CrossRef] [Google Scholar]
- Evaluation of the efficacy of intralesional measles, mumps, and rubella vaccine with intralesional vitamin D3 as immunotherapies in the treatment of recalcitrant cutaneous warts in adult- A randomized placebo-Controlled study. Indian Dermatol Online J. 2021;12:879-87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intralesional Vitamin D injection may be an effective treatment option for warts. J Cutan Med Surg. 2016;20:118-22.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of intralesional vitamin D3 in cutaneous warts: An open uncontrolled trial. J Cutan Aesthet Surg. 2017;10:90-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intralesional vitamin D3 injection in the treatment of recalcitrant warts: A novel proposition. J Cutan Med Surg. 2017;21:320-4.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of intralesional vitamin D3 injection in the treatment of cutaneous warts: A clinical therapeutic trial study. Skin Res Technol. 2023;29:e13442.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intralesional vitamin D3 versus intralesional purified protein derivative in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2019;32:e13034.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative evaluation of therapeutic response in warts to intralesional vitamin D3 versus intralesional measles, mumps, and rubella vaccine. Dermatol Ther. 2022;35:e15813.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study on the effectiveness of intralesional Measles, Mumps, and Rubella vaccine and intralesional vitamin D3 injection in the treatment of verruca. Our Dermatology Online. 2023;14
- [CrossRef] [Google Scholar]
- A randomised double blind active controlled study for comparison of intralesional MMR versus intralesional Vitamin D3 in the management of cutaneous warts. Assoc. Med. J. Volume.. 2021;1(1):24.
- [Google Scholar]
- Evaluation of efficacy and safety of intralesional vitamin D3 in comparison with intralesional measles, mumps and rubella (MMR) vaccine in the treatment of multiple cutaneous warts. Prz. Dermatol.. 2024;110:675-81.
- [Google Scholar]






