Translate this page into:
Intramatricial low-dose secukinumab injection for nail psoriasis
Corresponding author: Dr. ZhiQiang Yin, Department of Dermatology, First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu 210029, China. yinzhiqiang@njmu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: He F, Long F, Tu J, Yin Z. Intramatricial low-dose secukinumab injection for nail psoriasis. Indian J Dermatol Venereol Leprol 2021;87:116-9.
Sir,
Nail psoriasis affects not only the nail structure and function; it can also adversely impact the psychological state in psoriasis patients. What’s more, this disease lacks wholly effective treatment strategies. Secukinumab, an anti-IL-17A humanized monoclonal antibody, has been shown to cause good improvement in nail psoriasis in patients in whom it was given for moderate to severe plaque psoriasis. 1 However, in view of the adverse effect profile and costs, biologics are not the preferred drugs in treating isolated nail psoriasis. In this article, we propose a pilot therapy for nail psoriasis with intramatricial low-dose secukinumab injection.
Case 1 was a 33-year-old woman, weighing 44 kg with a BMI of 19.6 kg/m2, presenting with psoriasis vulgaris since 10 years and nail psoriasis since 3 years. She had repeatedly received intravenous systemic methotrexate (7.5 mg/week) and oral tripterygium glycosides for 3 months, which resulted in good control in skin psoriasis. However, there was no improvement in her 10 psoriatic nails and the nails took a turn for the worse, steadily, after withdrawing the drug 6 months back.
Case 2 was a 27-year-old woman weighing 53 kg, with a BMI of 22.1 kg/m2, suffering from psoriasis vulgaris for 6 years. She was intermittently treated with topical corticosteroids and calcipotriene in the past years. Two years back, she developed nail psoriasis involving all her 10 finger nails, but did not receive any specific treatment for the same. Both the patients had no history of chronic infections or malignancies.
Both the patients agreed to get treated with intramatricial secukinumab injection for nail psoriasis. The pilot therapy protocol was approved by the local ethics committee (2019-SR-421) and the informed consent to use their photographs was obtained from the patients. After local anesthesia with compound lidocaine cream 5% and tetracaine hydrochloride jelly 1% for an hour, intramatricial injection of low-dose secukinumab was administered. 2 In order to reduce local stimulation, secukinumab (150 mg/ml) was diluted with sterilized water for injection. The dosage was 0.1 ml diluted secukinumab per finger nail and the concentrations were 7.5, 15 and 30 mg/ml, respectively, from the second to fourth fingers of the left hand. The total dose was 5.25 mg every time and repeated every 2 weeks for 12 weeks. Meanwhile, there was no other systemic therapy or phototherapy or any topical therapy for the hands. The second to fourth fingers of the right hand were considered, a control group.
The severity of each nail was evaluated using the target nail psoriasis severity index scores, which allows a probable target nail score of 0 to 32.3 The baseline scores were 12, 10 and 8 for case 1 [Figure 1a], and 14, 12 and 12 for case 2 [Figure 2a]. The percentage improvement at week 6 relative to baseline from the second to fourth fingers of the left hand was 66.7%, 80% and 75% for case 1 [Figure 1b], and 35.7%, 58.3% and 50% for case 2 [Figure 2b]. The percentage improvement at week 12 relative to baseline of the left hand was 66.7%, 80% and 75% for case 1 [Figure 1c], and 42.9%, 75% and 50% for case 2 [Figure 2c]. Nail matrix and nail bed lesions both improved at the same time. Interestingly enough, all the untreated psoriatic nails of case 1 also had some improvement, and the percentage improvement of the right three finger nails was 25%, 37.5% and 66.7% at week 6, and 50%, 25% and 33.3% at week 12. Case 1 had very mild skin psoriasis with body surface area < 1% at baseline, and had no significant change at week 6 and 12. For case 2, the skin lesions and the untreated nails had no obvious change at week 6 and 12 compared with the baseline. We conducted a further 12-week follow-up. The percentage improvement at week 24 relative to baseline from the second to fourth fingers of the left hand was 91.7%, 90% and 75% for case 1 [Figure 1d], and 57.1%, 58.3% and 83.3% for case 2 [Figure 2d]. The percentage improvement at week 24 of the right three nails was 62.5%, 75% and 50% for case 1, and showed no significant improvement for case 2.
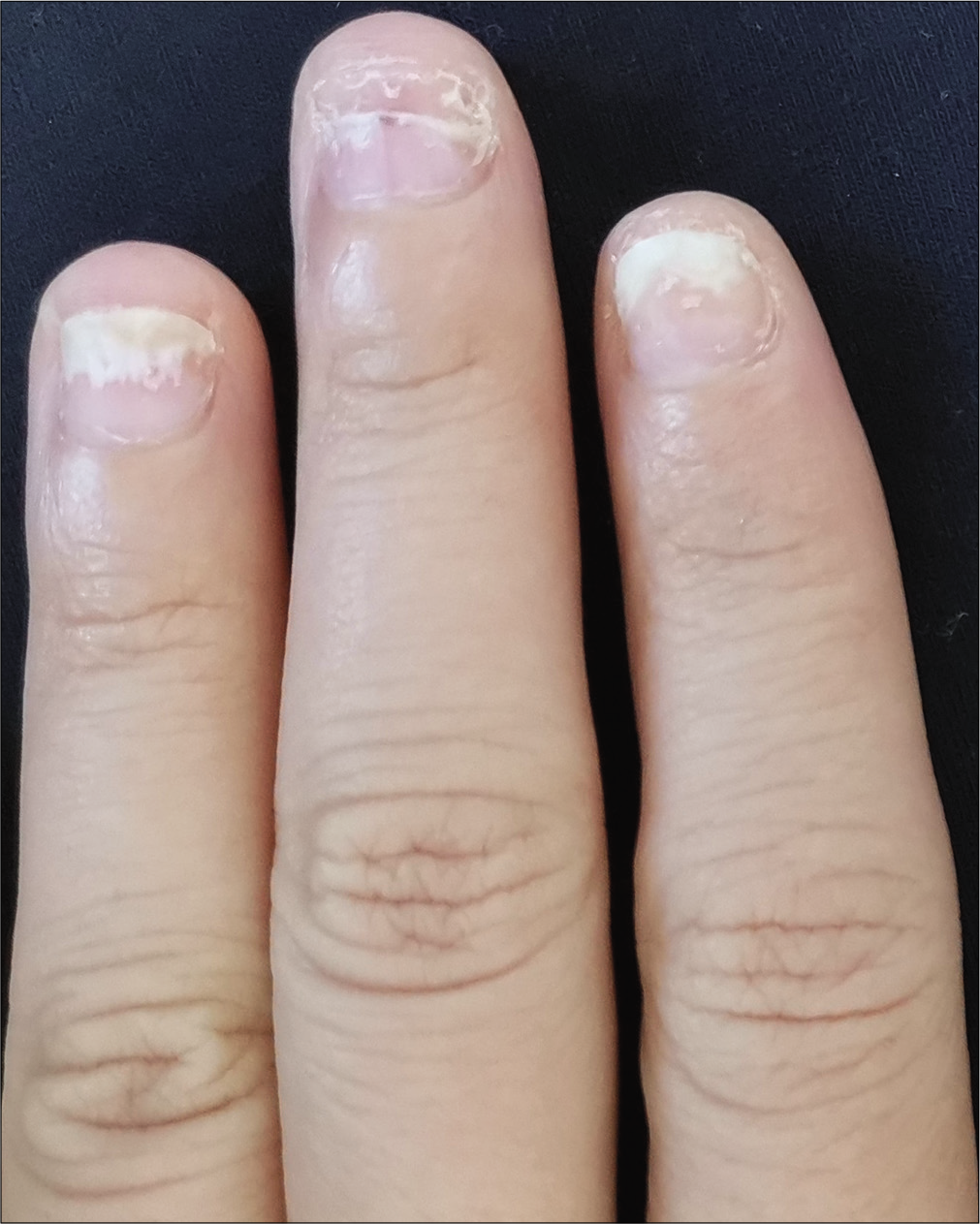
- Case 1 at baseline: The second to fourth fingers of the left hand before secukinumab intramatricial injection
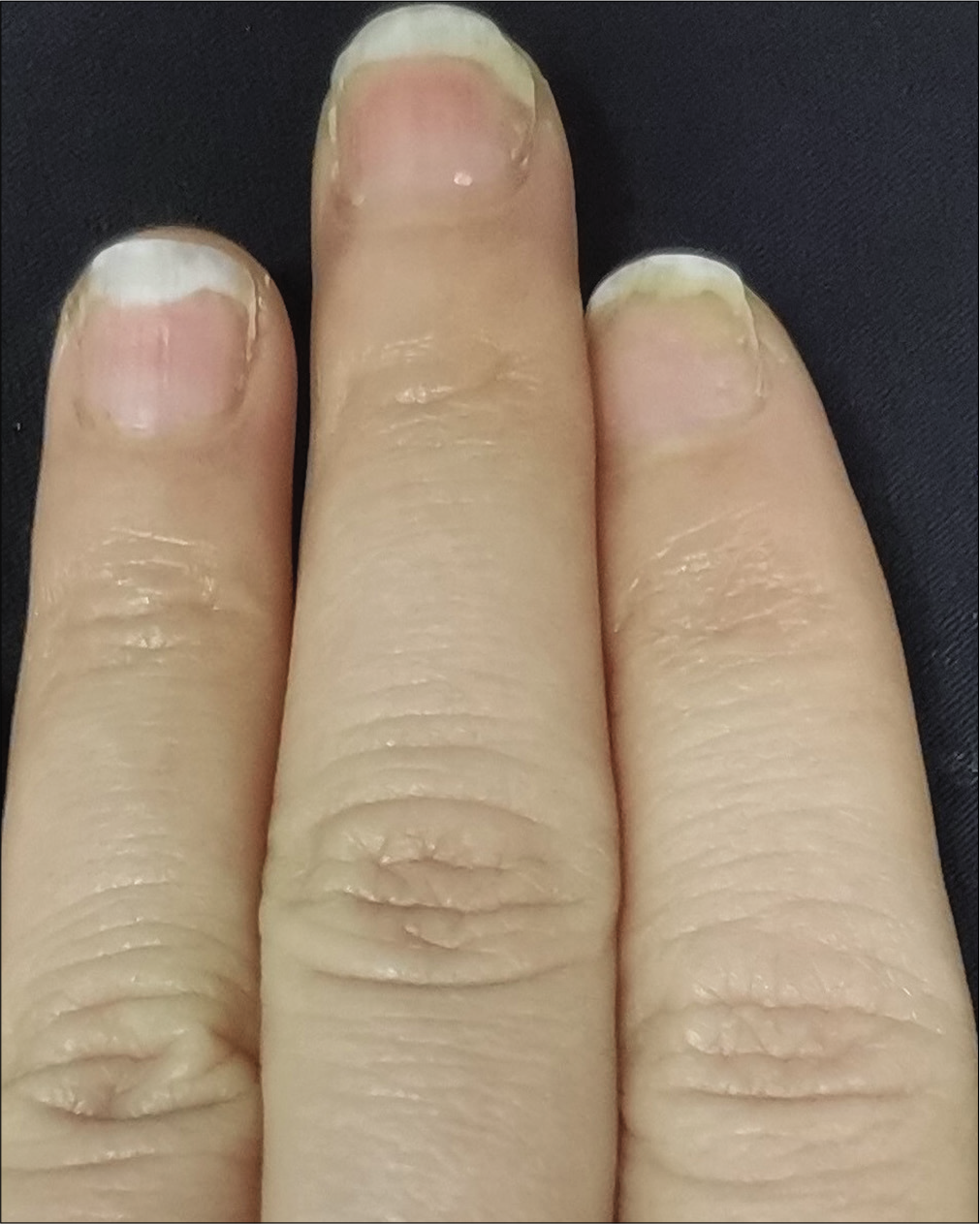
- Case 1 at week 6: Significant improvement observed after secukinumab intramatricial injection
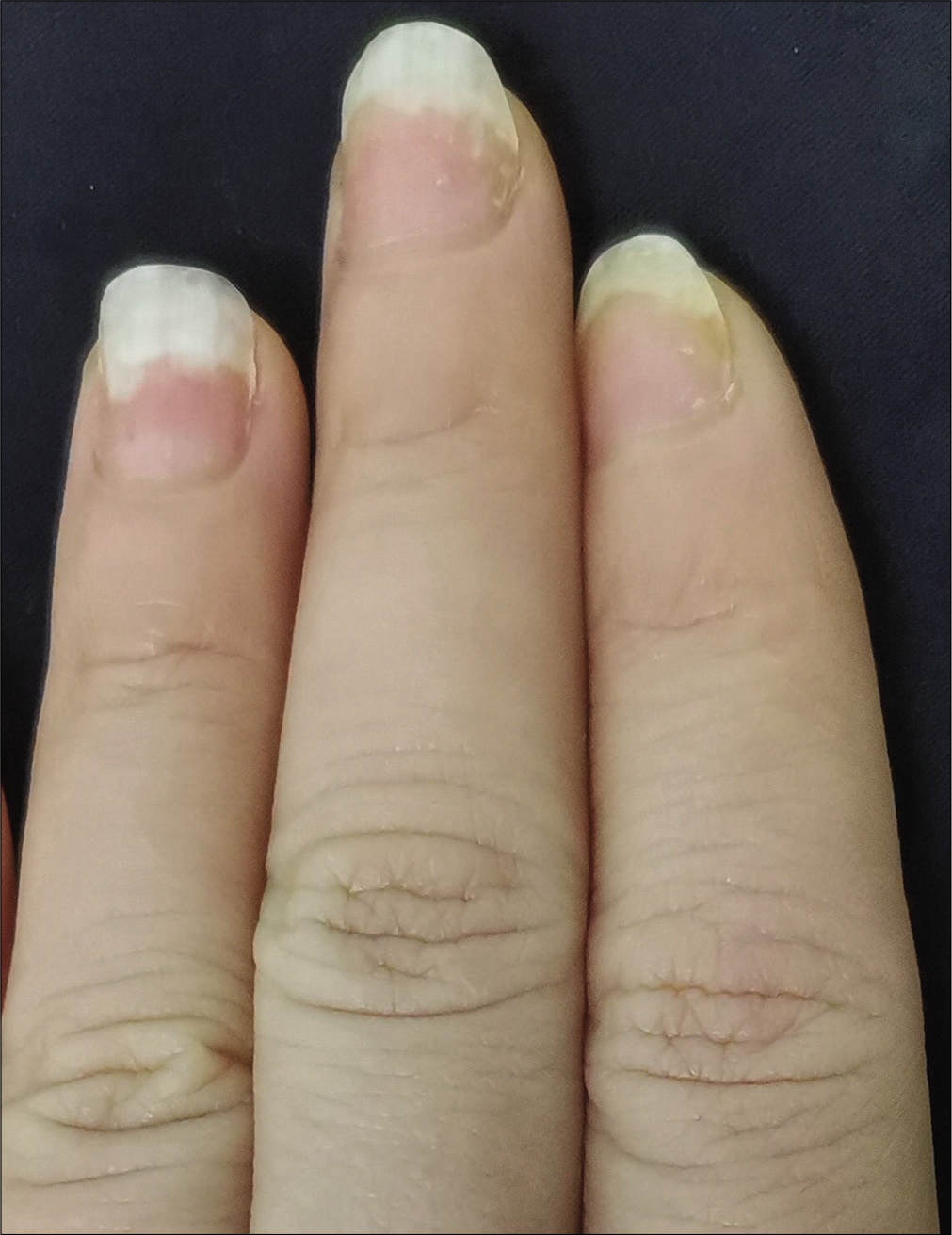
- Case 1 at week 12: Significant improvement observed after secukinumab intramatricial injection

- Case 1 at Week 24: Significant improvement observed after 12- week treatment and subsequent 12-week follow-up
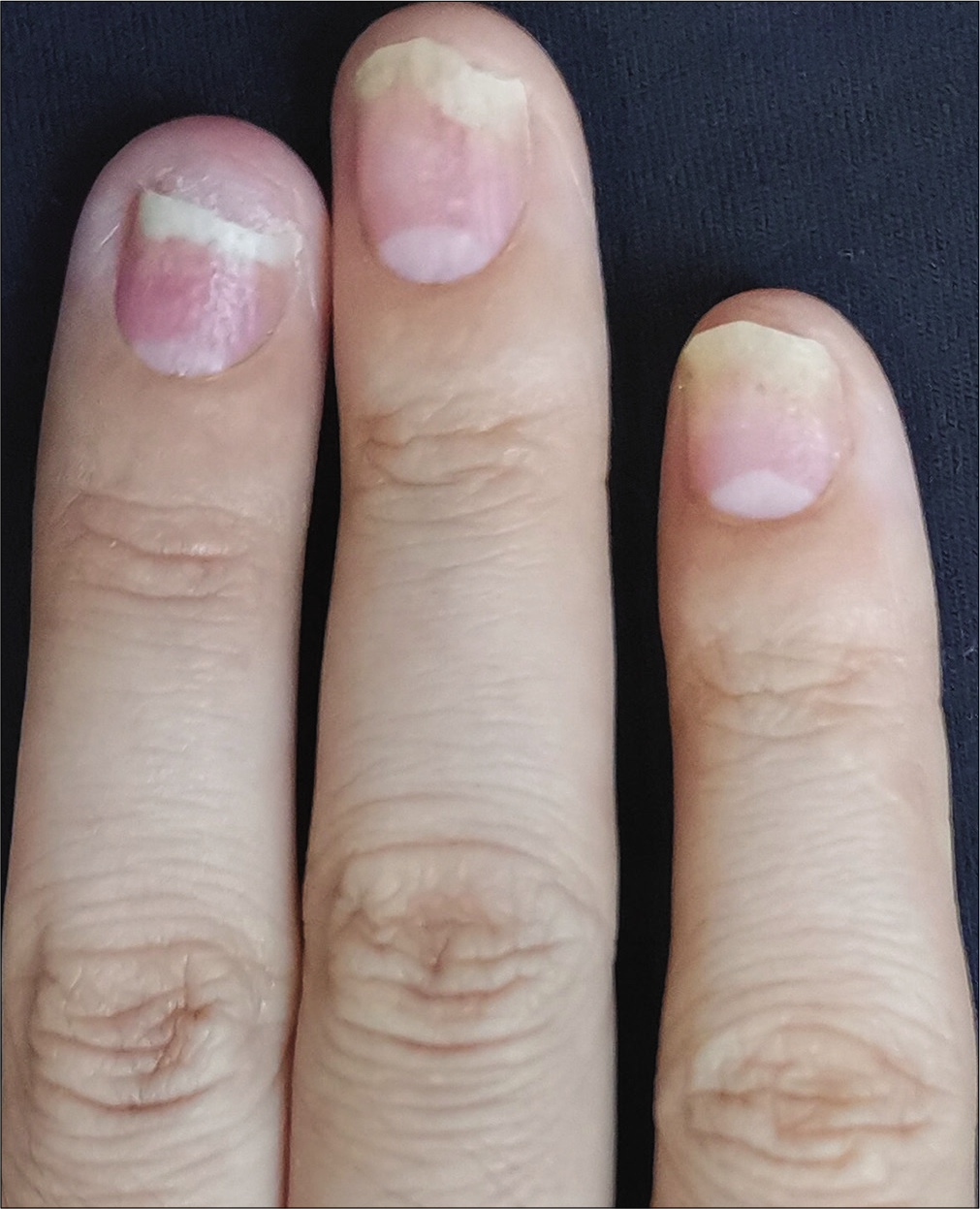
- Case 2 at baseline: The second to fourth fingers of the left hand before secukinumab intramatricial injection
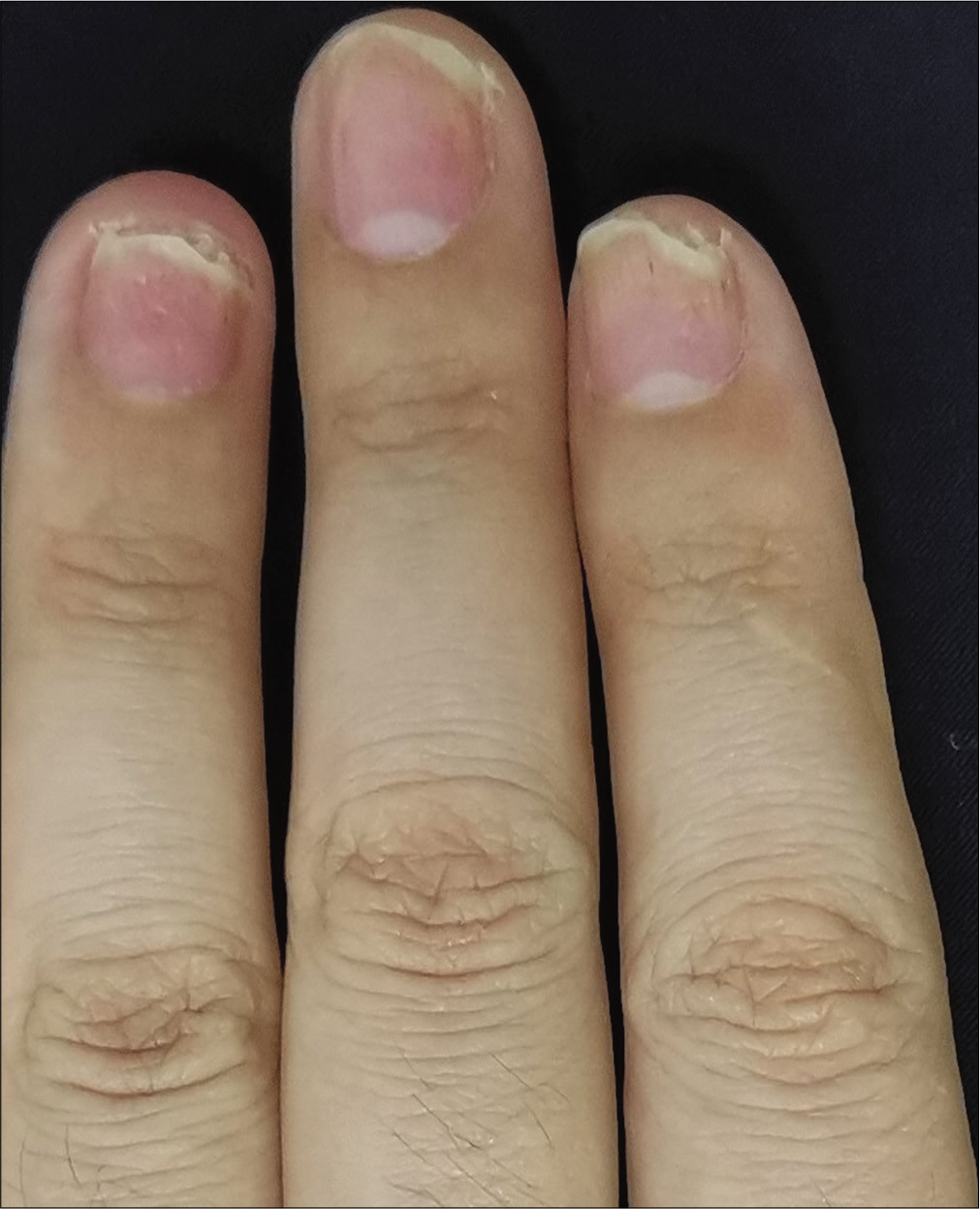
- Case 2 at week 6: Significant improvement observed after secukinumab intramatricial injection
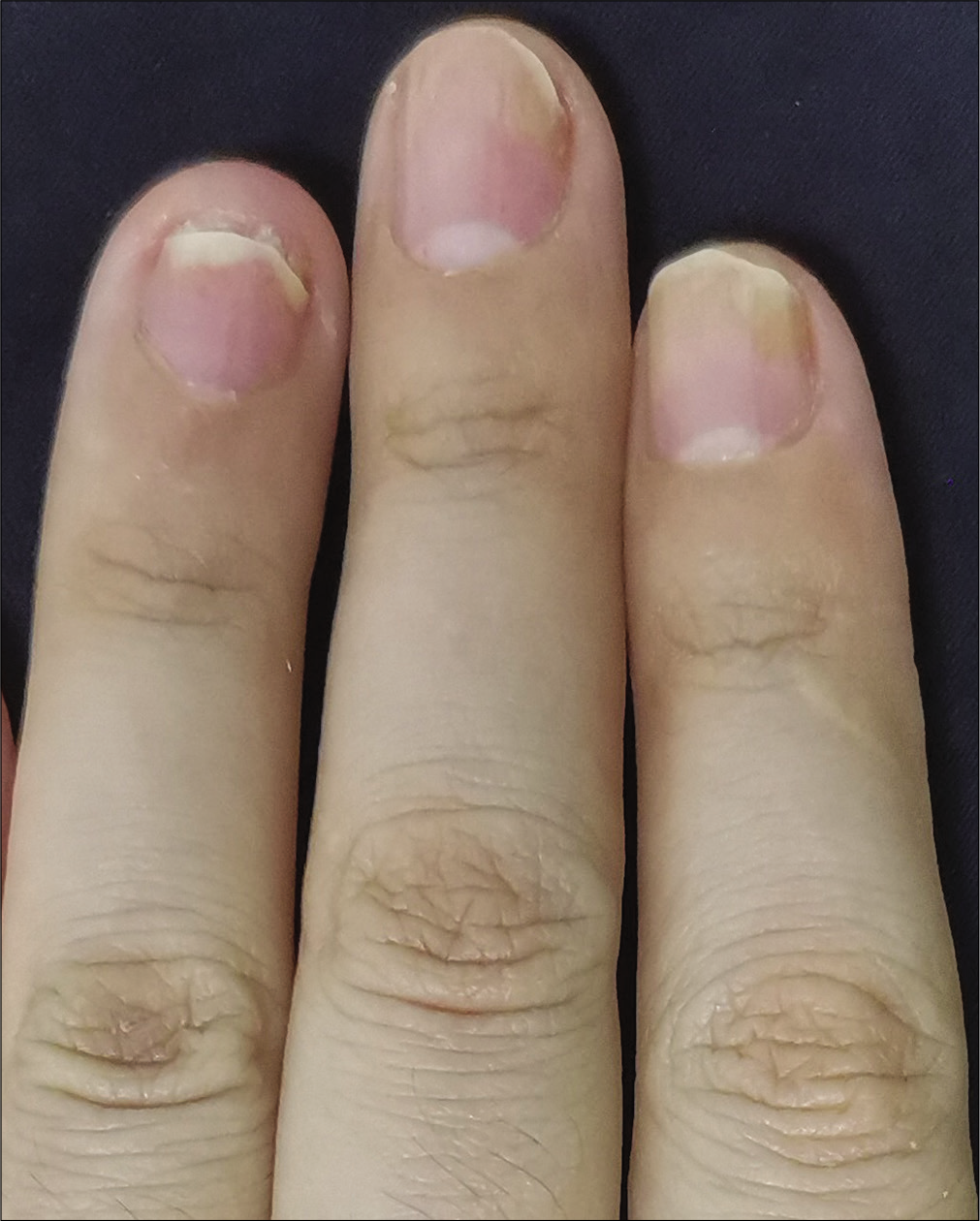
- Case 2 at week 12: Significant improvement observed after secukinumab intramatricial injection
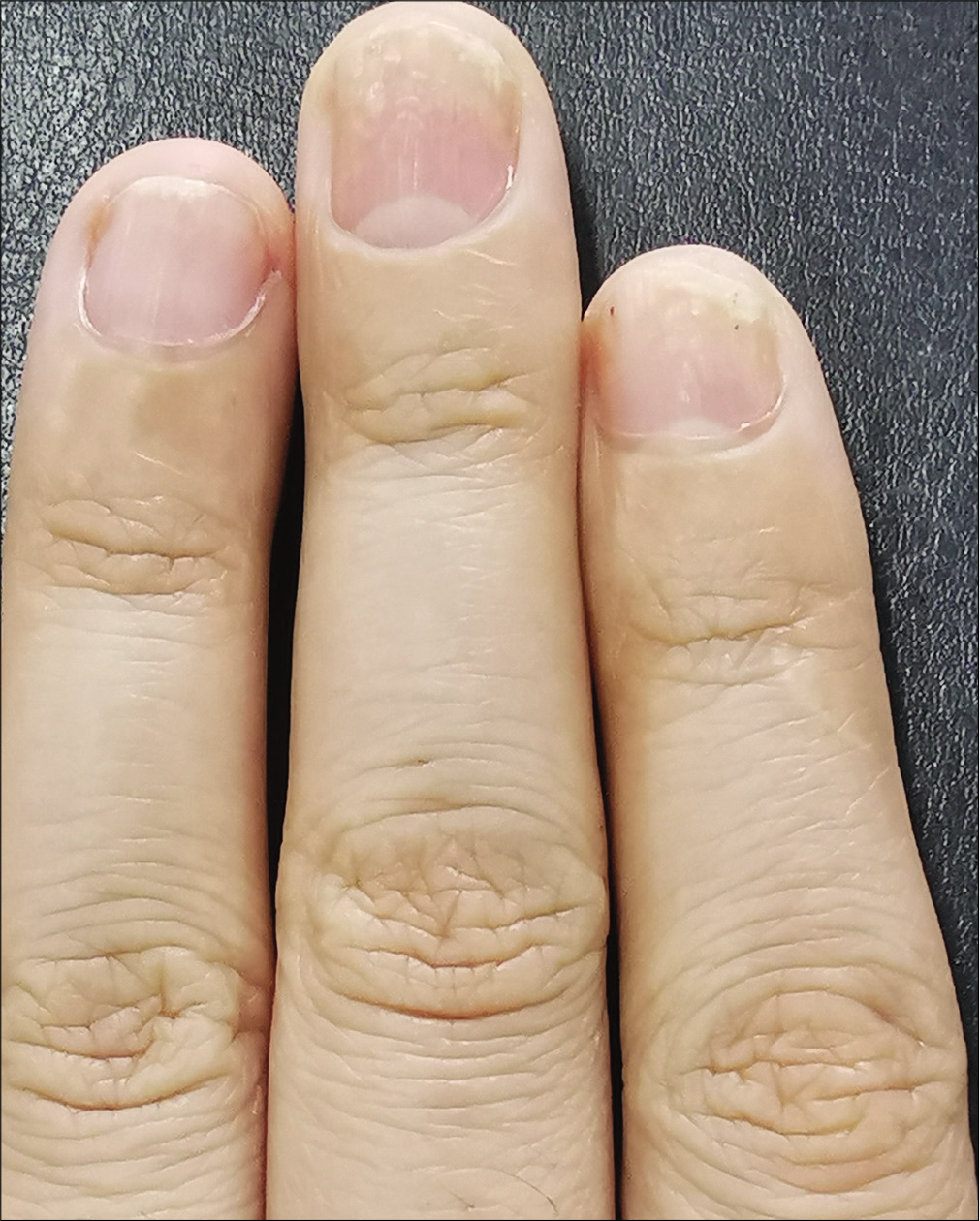
- Case 2 at week 24: Significant improvement observed after 12-week treatment and subsequent 12-week follow-up
Because the psoriatic nails of case 1 took a turn for the worse, steadily, before treatment, we thought the observed improvement in the untreated nails of case 1 could be attributed to systemic absorption and effects of the drug after low-dose secukinumab injection, especially considering her low body weight.
Both the patients felt transient and bearable pain while receiving injection, without any major discomfort. There were no events of tuberculosis or other infections. Full blood count, liver and renal functions were all within normal range at week 6 and 12.
This preliminary pilot therapy showed that low-dose secukinumab intramatricial injection seems to be a quick, effective and safe treatment method for nail psoriasis. The market price of secukinumab (150 mg) in China is about 420 dollars. Therefore, in our therapy, the estimated drug treatment cost for one psoriatic nail is 4.9 dollars each time approximately. Biopharmaceutical companies could design fractional packs for intramatricial injections, which should be placed at a low price, theoretically. Above all, the optimal dose, long-term efficacy and safety of intramatricial secukinumab injection need further large sample observation and validation.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (81673062). We thank Prof. Yan Lu for his help and suggestion to research design.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181:954-66.
- [CrossRef] [PubMed] [Google Scholar]
- Intramatricial injections for nail psoriasis: An open-label comparative study of triamcinolone, methotrexate, and cyclosporine. Indian J Dermatol Venereol Leprol. 2018;84:419-23.
- [CrossRef] [PubMed] [Google Scholar]
- Nail Psoriasis Severity Index: A useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49:206-12.
- [CrossRef] [Google Scholar]





