Translate this page into:
Intravenous methylprednisolone pulse therapy in severe alopecia areata
2 Department of Dermatology, University of Medicine and Pharmacy "I. Hatieganu", Cluj Napoca, Romania, Romania
Correspondence Address:
Rodica M Cosgarea
Department of Dermatology, Clinicilor Street 3-5, 400006, Cluj Napoca, Romania
Romania
| How to cite this article: Senila SC, Danescu SA, Ungureanu L, Candrea E, Cosgarea RM. Intravenous methylprednisolone pulse therapy in severe alopecia areata. Indian J Dermatol Venereol Leprol 2015;81:95 |
Abstract
Background: Severe, extensive, therapy resistant alopecia areata represents a clinical challenge. Systemic corticosteroids are a therapeutic tool that still needs to be evaluated. Aim: The purpose of this study was to assess the efficacy and safety of methylprednisolone pulse therapy in alopecia areata and to find prognostic factors for a favourable outcome. Methods: A total of 32 patients with severe multifocal alopecia areata (more than 40% scalp hair loss), alopecia totalis, and alopecia universalis were treated with infusions of 500 mg methylprednisolone for 3 days every month for 3 consecutive months. The end point of the study was 12 months. Results: Of 32 patients, 26 (81.3%) reported a clinical response. Four patients (12.5%) showed complete hair regrowth, 6 patients (18.8%) showed >50% hair regrowth, ten (31.3%) had <50% hair regrowth, 6 (18.75%) were non responders, and another 6 patients (18.8%) had relapse after an initial regrowth. Multivariate analysis revealed that patients reporting at the first episode and those with multifocal disease had the best results. Conclusion: Methylprednisolone infusions represent a possible therapeutic option for patients with multifocal alopecia areata and those presenting with the first episode of the disease.INTRODUCTION
Alopecia areata is a non-scarring, chronic, inflammatory disorder of the hair follicle of unknown etiology. It usually presents as sudden onset of limited, patchy, hair loss on the scalp that may progress and affect the entire scalp or other parts of the body. A trigger (e.g. viral infection, stressful event) may be identified 1-3 months before the onset of the disease. [1] It is difficult to predict the course of the disorder as local hair regrowth may be followed by relapse and new areas of hair loss can appear in previously unaffected sites. Several therapeutic options are available including potent topical or intralesional corticosteroids, topical immunotherapy (diphenylcyclopropenone, squaric acid dibutyl ester), anthralin, minoxidil, psoralen plus UVA (PUVA) phototherapy and phenolization. [2] However, all these treatments have a high failure rate, especially in persistent or extensive disease. The use of systemic corticosteroids in extensive alopecia areata is controversial due to the side effects associated with their prolonged oral administration. Therefore, the use of intravenous pulse corticosteroid therapy has been proposed to reduce adverse effects. [3] There are a few published studies that have assessed the efficacy of pulse therapy; however, different protocols, treatment regimens, endpoints, and hair loss evaluation methods make it difficult to draw any conclusions.
The aim of this study was to evaluate the effectiveness of methylprednisolone pulse therapy in patients with alopecia areata that is severe and resistant to topical therapies. Our intention was not only to monitor the efficacy and safety of the treatment and the relapse rate but also to evaluate the prognostic factors that have previously been published.
METHODS
The present study was a prospective, uncontrolled study in which patients with severe alopecia areata who were admitted between the years 2008 and 2012 to the Department of Dermatology at our institute were included. The study was approved by the Institute′s Ethics Committee.
Inclusion criteria : Patients with
- Multifocal, active alopecia areata that affected more than 40% of the scalp surface area
- Alopecia totalis and
- Alopecia universalis.
Exclusion criteria : Patients with contraindications to the use of systemic corticosteroids such as poorly controlled hypertension or diabetes mellitus, active infection, peptic ulcer etc.
Written informed consent was obtained from each patient or their legal representative. A detailed history and physical examination, complete and differential blood count, serum chemistry, electrolyte analysis, inflammation parameters, antinuclear antibodies, thyroid autoantibodies, and electrocardiography (EKG) were performed at baseline.
Treatment protocol and follow up
Treatment involved administration of 500 mg methylprednisolone infusion on 3 consecutive days. This was repeated at monthly intervals for 3 consecutive months. For patients under the age of 18 years, the dose of methylprednisolone was reduced to 6 mg/kg/day. Patients were monitored (including regular monitoring of pulse and blood pressure) during each infusion for any side effects of the therapy.
Efficacy evaluation
The efficacy of the treatment was evaluated monthly for 3 consecutive months and then at 6 and 12 months. Evaluation was based on observation of the hair regrowth in the bald lesions. Terminal hair growth was recorded as following: 100%, complete regrowth, more than 50%, significant regrowth (cosmetically acceptable), and less than 50%, minimal regrowth.
Relapse was defined as hair loss of at least 25% of the initially regrown hair and was recorded at a 12-months follow-up. In addition, pre and post treatment photographs of the specific area were also compared.
Hair regrowth was correlated with sex and the age of the patients, age of the onset, whether this was the first episode of alopecia areata, associated disorders, nail involvement, onset in the occipital area, type of alopecia (multifocal, totalis or universalis), duration of last episode (3-6 months, 7-12 months, over 12 months) and duration of disease (less than and more than 1 year). Statistical analysis was performed using Microsoft Excel and Statistical Package for the Social Sciences (SPSS) 20.0 (SPSS Inc., Chicago, IL, USA) software. The data were analyzed using Chi-square test and Cramer′s correlation coefficient, with P < 0.05 being statistically significant for all results.
RESULTS
Of the 32 patients included in the study, 12 were males (37.5%) and 20 were females (62.5%) with a mean age of 26.2 years (range: 4-59 years). The mean age of onset of disease was 21.8 years. The mean duration of disease was 4.3 years while the mean duration of the last episode was 12 months. Twenty-two of the 32 patients (68.8%) presented with multifocal alopecia areata, 2 (6.3%) had alopecia totalis, and the remaining 8 patients (25%) had alopecia universalis. Unsuccessful prior treatments included topical and intralesional corticosteroids, diphenylcyclopropenone, minoxidil and anthralin. Four patients (12.5%) had associated hypothyroidism and Hashimoto thyroiditis, 2 patients (6.3%) showed Hashimoto thyroiditis, 1 patient (3.1%) was diagnosed with chronic lupus erythematosus and 1 patient (3.1%) was diagnosed with both hypothyroidism and hypogonadism.
In 26 out of 32 patients (81.3%), a favorable clinical response was observed. Four out of 32 patients (12.5%) showed complete regrowth of scalp hair, six patients (18.8%) had more than 50% regrowth, ten patients (31.3%) had a regrowth less than 50%, and six patients (18.8%) had no response [Figure - 1]. Of the 26 responders, 6 patients (18.8%) showed a relapse at the 12 months of evaluation. The response to treatment regimen was significantly better in patients who presented with the first episode compared to those who had previously experienced hair loss (P = 0.041). Of the 22 patients who presented at the first flare of alopecia areata, four patients showed complete hair regrowth, six patients showed more than 50% regrowth, four patients had less than 50% regrowth, and five patients were non-responders [Figure - 2]. Three patients showed relapse at 12 months.
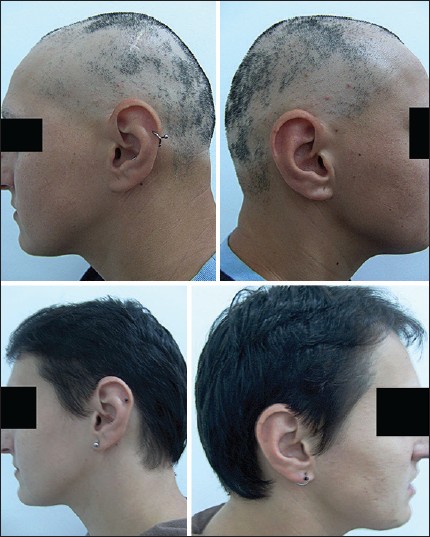 |
| Figure 1: Clinical picture of AA plaques at baseline and complete hair regrowth at 12 month endpoint |
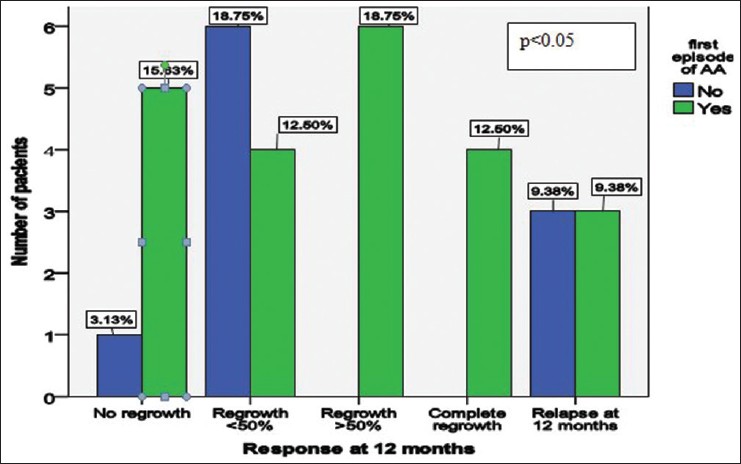 |
| Figure 2: Treatment response at 12 month endpoint for patients at the first episode of alopecia areata compared with the rest of the group |
The treatment response was also influenced by the type of alopecia with patients with multifocal alopecia areata showing a statistically significant better response than patients with alopecia totalis and alopecia universalis (P = 0.049). Of 22 patients with multifocal alopecia areata, two patients had complete regrowth, five patients had significant regrowth, eight patients had minimal regrowth while two patients were non-responders. Of the responders, five patients had a relapse of the disease. Treatment response was much lower for patients with alopecia universalis and alopecia totalis. Six of 8 patients with alopecia universalis showed unsatisfactory regrowth (minimal, no response or relapse). Only two (6.25%) patients with alopecia totalis were included in the study, one showing total hair regrowth and one showing no response [Figure - 3].
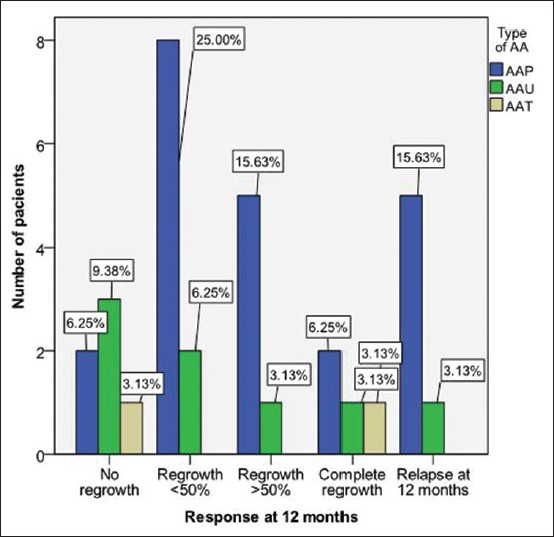 |
| Figure 3: Treatment response at 12 month endpoint according to type of alopecia areata |
The onset of the last episode influenced the response to therapy. Of 21 patients who experienced their last episode of disease exacerbation in the preceding one year, three showed complete response, six significant response, seven minimal response while five were non-responders. Of the responders, five showed relapse at the 12 months evaluation [Figure - 4].
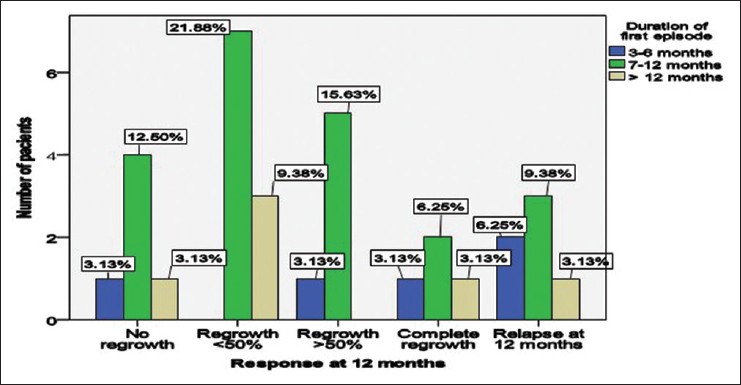 |
| Figure 4: Clinical response according to duration of the first episode of alopecia areata |
Duration of disease also influenced the outcome of the treatment. Of the 13 patients with total duration of disease of less than one year, complete hair regrowth was only noted in four patients, significant hair regrowth in three patients, and minimal and no response was noted in three patients each. None of the patients with disease duration more than one year experienced complete hair regrowth [Figure - 5]. Of the 5 patients in whom the initial site of involvement was the occipital region, one patient had minimal regrowth, one patient had significant regrowth, no patient had complete hair regrowth while three were non-responders. At the 12 month evaluation, two (6.25%) patients had a relapse. The patients with nail changes of trachyonychia did not respond to therapy. None of the 8 patients with associated diseases completely responded to therapy, one patient presented more than 50% hair regrowth while three patients presented minimal hair regrowth. However, 2 patients had relapse at 12 months evaluation. Treatment outcome was not statistically influenced by the sex (P = 0.19) or age of the patients (P = 0.61), the age of the onset (P = 0.51), the associated diseases (P = 0.71), the duration of the last episode (P = 0.69), or the duration of the disease (P = 0.18).
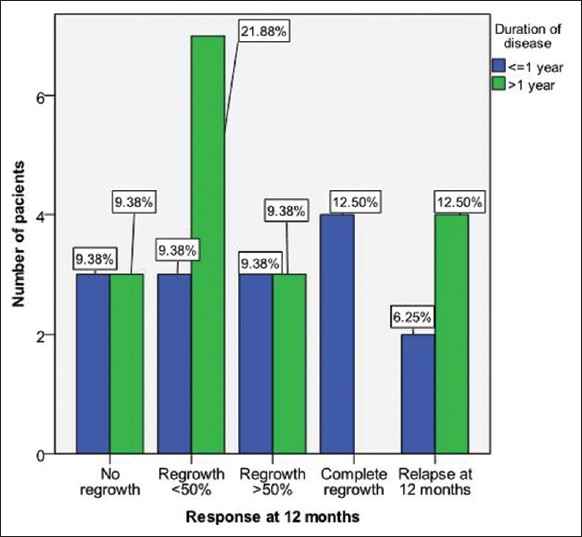 |
| Figure 5: Clinical response according to duration of disease |
Treatment side effects included headache and dizziness in nine patients (28.1%), facial flushing in seventeen patients (53.1%), asthenia in five patients (15.6%), and palpitation in six patients (18.8%). No major side effects requiring cessation of therapy were registered.
DISCUSSION
Multifocal, progressive, therapy-resistant alopecia areata represents a challenge for the treating dermatologist despite the effort that has been made in understanding the pathogenesis and evolution of the disease. Since the underlying pathology involves aberrant cell-mediated immune response including lymphocyte mediated inflammation, [4] it has led to the development of treatments that have an immunomodulatory mechanism. High-dose corticosteroid pulse therapy represents a treatment option for severe alopecia areata, even though the data supporting its effectiveness is limited and further evidence is needed to assess the benefit/risk ratio of this therapy.
Systemic corticosteroids have been proven to induce hair regrowth, however, treatment relapse and therapy side effects limit this approach. Olsen et al., [5] obtained more than 25% hair regrowth after a 6-weeks oral prednisone regimen which was maintained with topical application of 2% minoxidil at the 20-weeks assessment. Side effects were considered to be acceptable. Although Kar et al. noted considerable hair regrowth in patients treated with 200 mg oral prednisolone compared to placebo control at 6 months, [6] Sladden et al. suggested that results need to be validated by further such studies. [7] On the other hand, a small study performed by Kalin and Hunziker [8] needed to be discontinued due to side effects of oral corticosteroid pulse therapy with authors reporting only one out of ten patients as having stable, satisfactory hair regrowth.
Intravenous corticosteroid pulse therapy represents a viable alternative to oral administration due to fewer side effects and relative ease of administration. Our results are consistent with the previously published data as multifocal alopecia areata responded better to systemic steroids than alopecia totalis and alopecia universalis. Similar results were also published by Seiter et al.[9] who treated 30 patients with intravenous methylprednisolone (8 mg/body weight for three days) with more than 50% hair regrowth in 67% of patients with multifocal alopecia areata. However, none of the patients with alopecia totalis and alopecia universalis responded to therapy. A 3-day treatment regimen with 250 mg of methylprednisolone twice daily was administered by Friedli et al., [10] with a good response rate in patients with multifocal disease. Interestingly, almost half of the patients with alopecia totalis showed a delayed but significant response to the treatment. Results for patients with alopecia universalis were less encouraging.
We used the same protocol as previously published by Luggen and Hunziker, [11] which was well tolerated by patients. An adjusted dose of 8 mg/body weight has also been used [12] and a regimen of 100 mg prednisolone, following the same protocol. [13] Other regimens of systemic corticosteroids include oral dexamethasone 0.5 mg/day, intramuscular triamcinolone acetonide 40 mg once a month and oral predonine 80 mg for 3 consecutive days once a month for 6 months. [14]
Im et al., [15] reported that treatment outcome was influenced by the type of alopecia areata and duration of disease; disease duration of 6 months or less before initiation of corticosteroid pulse therapy was associated with a better response to intravenous therapy. Similar results were also obtained by Nakajima et al., [16] in a study on 139 patients: a good response to pulse therapy (defined as regrowth of more than 75%) was noted in 59.4% and 15.8% of patients with disease less than or more than 6 months, respectively. Our findings are thus in concordance with previous reports.
Even though the tendency of hair regrowth was higher in patients with disease duration less than 12 months than in patients with a longer duration, the results did not achieve statistical significance. Similar results were observed while analyzing for the progression of the disease: patients with a longer history of disease had a less favorable treatment response compared to patients with a shorter history of the disease. Statistically significant results were not obtained on further analysis.
The age of onset is another factor to consider, even though different studies have used different cut-offs. In a large study of 1030 patients, disease onset below 16 years of age was associated with a lower cure rate, while a long disease duration was associated with a higher risk of relapse. [17] A study performed in China observed an association between the onset of alopecia areata before 30 years of age and both the severity of the disease and its duration. [18] In a study including patients <16 years of age, it was observed that the earlier the age of onset, the more severe was the alopecia areata. [19] However, a study of patients with alopecia totalis and alopecia universalis found no statistically significant association between hair loss and treatment modalities in patients with disease onset below and above 13 years of age. [20] We did not find a statistically significant relationship between age of onset and treatment response.
Alopecia totalis and alopecia universalis usually show a poorer response to corticosteroid pulse therapy. [21] In a study using an intravenous treatment regimen of methylprednisolone, 8 mg/kg body weight, Friedland et al., [22] found that multifocal alopecia areata responded better to treatment than alopecia totalis, alopecia universalis or multifocal alopecia with ophiasis. In another study, a low efficiency of methylprednisolone bolus for alopecia totalis and universalis was noted at 6 months and after a mean of 12.3 years follow up. [23]
Relapse and lack of hair regrowth after topical treatments represent a reason for the clinician to continue the search for additional therapeutic options. Even though no significant side effects of methylprednisolone pulse therapy in alopecia areata have yet been reported, one must always be cautious, monitor patients carefully and expect a positive benefit/risk ratio. Our study suggests that intravenous methylprednisolone therapy might be useful for patients resistant to topical therapies ; however, these findings should be validated in studies with a larger number of subjects. A guideline for enhancing study accuracy for alopecia areata has already been published [24] but, in this case, a placebo-controlled study would be extremely difficult to perform since patients are already disappointed by previous treatment modalities and fearful about losing the remaining hair. It is nearly impossible to compare methylprednisolone infusions with a best current therapeutic method since patients included in our study had already been treated with a wide range of topical therapies.
In conclusion, our results suggest that infusions with methylprednisolone may represent an option for patients with multifocal alopecia areata who are resistant to topical therapies and who need professional treatment at the first episode of hair loss. There is a need for further data in order to obtain an evidence-based treatment for alopecia areata, as well as for new treatment modalities for refractory cases.
| 1. |
Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol 2013;2013:348546.
[Google Scholar]
|
| 2. |
Kar S, Singh N. Alopecia areata treated with phenolisation and intravenous dexamethasone pulses. Int J Trichology 2013;5:47-9.
[Google Scholar]
|
| 3. |
Burton JL, Shuster S. Large doses of glucocorticoid in the treatment of alopecia areata. Acta Derm Venereol 1975;55:493-6.
[Google Scholar]
|
| 4. |
Wolff H. Diseases of hair. In: Burgdorf WH, Plewig G, Wolff HH, Landthaler M, editors. Braun Falco's Dermatology. 3 rd ed. Heidelberg: Springer Medizin Verlag; 2009. p. 1050-2.
rd ed. Heidelberg: Springer Medizin Verlag; 2009. p. 1050-2.'>[Google Scholar]
|
| 5. |
Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol 1992;128:1467-73.
[Google Scholar]
|
| 6. |
Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol 2005;52:287-90.
[Google Scholar]
|
| 7. |
Sladden MJ, Hutchinson PE. Is oral pulsed prednisolone useful in alopecia areata? Critical appraisal of a randomized trial. J Am Acad Dermatol 2005;53:1100-1.
[Google Scholar]
|
| 8. |
Kalin U, Hunziker T. High-dose intravenous corticosteroid pulse therapy in alopecia areata: Own experience compared with the literature. J Dtsch Dermatol Ges 2008;6:895-6.
[Google Scholar]
|
| 9. |
Seiter S, Ugurel S, Tilgen W, Reinhold U. High-dose pulse corticosteroid therapy in the treatment of severe alopecia areata. Dermatology 2001;202:230-4.
[Google Scholar]
|
| 10. |
Friedli A, Labarthe MP, Engelhardt E, Feldmann R, Salomon D, Saurat JH. Pulse methylprednisolone therapy for severe alopecia areata: An open prospective study of 45 patients. J Am Acad Dermatol 1998;39:597-602.
[Google Scholar]
|
| 11. |
Luggen P, Hunziker T. High-dose intravenous corticosteroid pulse therapy in alopecia areata: Own experience compared with the literature. J Dtsch Dermatol Ges 2008;6:375-8.
[Google Scholar]
|
| 12. |
Ito T. Advances in the management of alopecia areata. J Dermatol 2012;39:11-7.
[Google Scholar]
|
| 13. |
Efentaki P, Altenburg A, Haerting J, Zouboulis CC. Medium-dose prednisolone pulse therapy in alopecia areata. Dermatoendocrinol 2009;1:310-3.
[Google Scholar]
|
| 14. |
Kurosawa M, Nakagawa S, Mizuashi M, Sasaki Y, Kawamura M, Saito M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology 2006;212:361-5.
[Google Scholar]
|
| 15. |
Im M, Lee SS, Lee Y, Kim CD, Seo YJ, Lee JH, et al. Prognostic factors in methylprednisolone pulse therapy for alopecia areata. J Dermatol 2011;38:767-72.
[Google Scholar]
|
| 16. |
Nakajima T, Inui S, Itami S. Pulse corticosteroid therapy for alopecia areata: Study of 139 patients. Dermatology 2007;215:320-4.
[Google Scholar]
|
| 17. |
Uchiyama M, Egusa C, Hobo A, Irisawa R, Yamazaki M, Tsuboi R. Multivariate analysis of prognostic factors in patients with rapidly progressive alopecia areata. J Am Acad Dermatol 2012;67:1163-73.
[Google Scholar]
|
| 18. |
Yang S, Yang J, Liu JB, Wang HY, Yang Q, Gao M, et al. The genetic epidemiology of alopecia areata in China. Br J Dermatol 2004;151:16-23.
[Google Scholar]
|
| 19. |
Xiao FL, Yang S, Liu JB, He PP, Yang J, Cui Y, et al. The epidemiology of childhood alopecia areata in China: A study of 226 patients. Pediatr Dermatol 2006;23:13-8.
[Google Scholar]
|
| 20. |
Cho HH, Jo SJ, Paik SH, Jeon HC, Kim KH, Eun HC, et al. Clinical characteristics and prognostic factors in early-onset alopecia totalis and alopecia universalis. J Korean Med Sci 2012;27:799-802.
[Google Scholar]
|
| 21. |
Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists' guidelines for the management of alopecia areata 2012. Br J Dermatol 2012;166:916-26.
[Google Scholar]
|
| 22. |
Friedland R, Tal R, Lapidoth M, Zvulunov A, Ben Amitai D. Pulse corticosteroid therapy for alopecia areata in children: A retrospective study. Dermatology 2013;227:37-44.
[Google Scholar]
|
| 23. |
Staumont-Sallé D, Vonarx M, Lengrand F, Segard M, Delaporte E. Pulse corticosteroid therapy for alopecia areata: Long-term outcome after 10 years. Dermatology 2012;225:81-7.
[Google Scholar]
|
| 24. |
Olsen EA. Investigative guidelines for alopecia areata. Dermatol Ther 2011;24:311-9.
[Google Scholar]
|
Fulltext Views
4,033
PDF downloads
1,541





