Translate this page into:
Iontophoresis in dermatology
Correspondence Address:
C R Srinivas
Department of Dermatology, PSG Hospitals, Peelamedu, Coimbatore - 641 004, Tamil Nadu
India
| How to cite this article: Rai R, Srinivas C R. Iontophoresis in dermatology. Indian J Dermatol Venereol Leprol 2005;71:236-241 |
Abstract
Iontophoresis is the process of increasing the penetration of drugs into the skin by application of an electric current. The drug is applied under an electrode of the same charge as the drug, and a return electrode opposite in charge to the drug is placed at a neutral site on the body surface. Electrical energy assists the movement of ions across the skin using the principle "like charges repel each other and opposite charges attract". In this article, we discuss the mechanism, principles, factors influencing iontophoresis and its application for various dermatological conditions.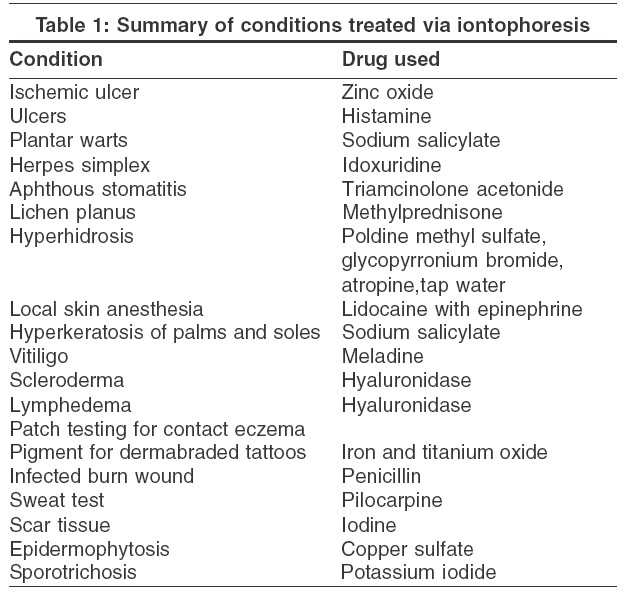

 |
 |
Introduction
Trans-dermal administration of drug is assuming an important place in modern drug therapy. It is used for non-ionized drugs required in a small dosage. Trans-dermal administration can be passive or facilitated. In passive administration, the non-ionized drug traverses the skin through the stratum corneum. The skin, being a semi-permeable membrane, allows only a small amount of any drug molecule to passively penetrate the skin.[1] Ionized drugs do not easily penetrate this barrier and are not suitable for routine trans-dermal delivery unless an external source of energy is provided to drive the drug across the skin. Facilitated diffusion can utilize either ultrasound (phonophoresis) or electrical (iontophoresis) energy. In iontophoresis, this external source of energy is in the form of an applied direct electrical current.
Principles of iontophoretic treatment
Iontophoresis increases the penetration of electrically charged drugs into surface tissues by the application of an electric current.[2] Electrical energy assists the movement of ions across the stratum corneum according to the basic electrical principle of "like charges repel each other and opposite charges attract".
The drug is applied under an electrode of the same charge as the drug, and a return electrode opposite in charge to the drug is placed at a neutral site on the body surface. The operator then selects a current below the level of the patient′s pain threshold and allows it to flow for an appropriate length of time. The electrical current significantly increases the penetration of the drug into surface tissues by repulsion of like charges and attraction of opposite charges. The two classically considered prerequisites for iontophoretic treatment are that the drug must be charged (or modified to carry a charge) and that the disease process must be at or near a body surface.
Iontophoresis mechanism and devices
A typical iontophoresis device consists of DC voltage delivery system and electrodes. Wires are then connected between the unit and the active and passive electrodes, and the unit set for current and time [Figure - 1]. In the iontophoresis process, the current, beginning at the device, is transferred from the electrode through the ionized drug solution as ionic flow. The drug ions are moved to the skin where the repulsion continues moving the drug through the trans-appendageal structures and stratum corneum interstices via the aqueous pores.[3] The larger the electrode surface, the greater the current the device must supply to provide a current density for moving the drug.
Iontophoresis enhances transdermal drug delivery by three mechanisms: (a) ion-electric field interaction provides an additional force that drives ions through the skin, (b) the flow of electric current increases the permeability of the skin, and (c) electro-osmosis produces bulk motion of solvent that carries ions or neutral species with the solvent stream. Electro-osmotic flow occurs in a variety of membranes and is in the same direction as the flow of counter-ions. It may assist or hinder drug transport. Since human skin is negatively charged above pH 4, counter ions are positive ions and electro-osmotic flow occurs from anode to cathode. Thus, anodic delivery is assisted by electro-osmosis but cathodic delivery is retarded. Because of the electro-osmotic flow, transdermal delivery of a large anion (negatively charged protein) from the anode compartment is more effective than that from the cathode compartment.[4]
Iontophoretic devices may be powered by electricity, batteries or by rechargeable power sources. The machines available in India are electric operated. Battery operated units are Drionicγ, Phoresorγ, etc. A Phoreserγ device consists of a microprocessor-controlled battery powered DC current, drug reservoir and electrodes. The batteries are most commonly 9 volt ones. The drug reservoir consists of a gauze/cloth or gel pad to which the solution is applied or the solution is injected through a port into the reservoir electrode combination. Wires are connected between the microprocessor unit and the active and passive electrodes. Iomed iontophoretic drug delivery electrodes are available and are composed of hydrogel material that is hydrated before use to deliver local anesthesia.
Reverse iontophoresis
The application of an electric current across the skin is used to extract a substance of interest from within or beneath the skin to the surface for testing. In vivo and in vitro methods have shown that the approach can be used to monitor the subdermal concentration variation of glucose and provide a non-invasive technique by which a diabetic can monitor the fluctuation of blood sugar.[5]
Advantages
Iontophoresis provides for controlled delivery rates (through variations of current density, pulsed voltage, drug concentration and ionic strength). It eliminates gastrointestinal incompatibility, erratic absorption, and first pass metabolism. It reduces side effects and avoids the risks of infection, inflammation, and fibrosis associated with continuous injection or infusion since it is non-invasive. It enhances patient compliance with a convenient and non-invasive therapeutic regimen, and decreased dosing frequency. It improves efficacy by continuous release and decreases the total dose and dosing frequency.
Disadvantages
There is a possibility of burns if the electrodes are improperly used. The formation of undesirable vesicles and bullae in skin being treated can be avoided by periodically interrupting a unidirectional treatment current with a relatively short pulse of current in the opposite direction.
Factors affecting iontophoresis
Variables affecting the iontophoresis include aspects of the current, the physicochemical properties of the drug, formulation factors, biological factors and electro-endo-osmotic flow.
Current
The current can be direct, alternate or pulsed, and can have various waveforms, including square, sinusoidal, triangular and trapezoidal. The more complex forms may not be of much advantage as direct current is most commonly used. In a recent study, alternating current (AC) iontophoresis showed better results than conventional constant current DC iontophoresis. Constant conductance AC iontophoresis showed reduced flux drift and less skin to skin variability compared to conventional constant current DC iontophoresis.[6]
Physicochemical variables
These include the charge, size, structure and lipophilicity of the drug. The drug should be water soluble, low-dose and ionizable with a high charge density. Smaller molecules are more mobile but large molecules are also iontophoresable.
Formulation factors
These include the drug concentration, pH, ionic strength, and viscosity.
1. Drug concentration: Increasing drug concentrations results in greater drug delivery to a certain degree.
2. Ionic strength: If buffer ions are included, they compete with the drug for the delivery, decreasing the quantity of drug delivered, especially since buffer ions are generally smaller and more mobile than the larger active drug.
3. pH: The pH of the solution can be adjusted and maintained by larger molecules, such as ethanolamine: ethanolamine hydrochloride rather than the smaller hydrochloric acid and sodium hydroxide. An increase in ionic strength of the system will also increase the competition for the available current, especially since the active drugs are generally potent and present in a smaller concentration than these extraneous ions.
4. Viscosity: The migration of the drug is inversely related to the viscosity.
Biological factors
These factors involve the skin to which the electrodes are applied; its thickness, permeability, presence of pores, etc. Sweat glands are the most significant path for the conduction of charges into the skin. This was demonstrated by Papa and Kligman, when methylene blue introduced into the skin via iontophoresis entered sweat glands in a punctuate pattern and outlined the sweat pores.[7]
Electro-endo-osmosis transport
Drugs can also be transported via electro-endo-osmosis. When a current is applied, there is also a flow of water from the electrode reservoir into the skin. Any drug in solution, ionized or non-ionized, can follow the water flow into the skin. In this manner, some drugs that are not ionized can also be given iontophoretically. If only the non-ionized drug is to be given, it may be necessary to add a small quantity of sodium chloride to the solution for conductivity and to establish electro-endo-osmotic flow.
Dermatologic applications of iontophoresis
Iontophoresis has been used for the treatment of various dermatologic conditions [Table - 1]. The majority of published studies are either uncontrolled series or anecdotal observations. Earlier, simple ions and heavy metals were the most frequently used drugs, but over the last 30 years interest has shifted toward the use of iontophoresis as a drug delivery system for a wide variety of medications, ranging from steroids to antibiotics to local anesthetics.
Ulcers Iontophoresis has been used for the treatment of patients with ischemic leg ulcers. The effect of histamine iontophoresis on ulcers was studied by Abramson et al.[8] Complete healing was reported in four of the five patients.[8] Cornwell reported a patient with ischemic ulcer who responded to iontophoresis with solution of zinc oxide.[9]
Fungal infection There are reports of the successful treatment of dermatophytosis with the use of copper sulfate iontophoresis[10] and of sporotrichosis with potassium iodide iontophoresis.[11]
Viral Infections
Warts: There is a report of the successful treatment of plantar warts with sodium salicylate iontophoresis.[12]
Herpes simplex: Gangarosa reported that iontophoretic application of idoxuridine was effective in aborting episodes of herpes simplex.[13] Lekas reported relief of discomfort and reduction of healing time of herpes simplex lesions in a controlled trial using iontophoresis with idoxuridine .[13]
Aphthous stomatitis In a small group of patients with aphthous stomatitis, iontophoresis of triamcinolone acetonide showed immediate relief of discomfort in the prodromal stage, but for lesions beyond the prodromal stage relief was not achieved until after 36 hours.[14]
Lichen planus In an uncontrolled series, Gangarosa reported iontophoresis with methyl prednisolone for erosive lichen planus, which healed with fibrosis.[15]
Hyperhidrosis The most successful application of iontophoresis is for the treatment of hyperhidrosis. The basis for such treatment and its practical aspects have been well described.[16],[17],[18] Currently, the most commonly used conducting medium is tap water because it is safe and effective.[19] Anticholinergic compounds (e.g. poldine methyl sulfate,[20] glycopyrronium bromide,[21] and atropine[22]) have a longer lasting effect than water, but the side effects of systemic anticholinergic blockade have prevented their wide acceptance.
The efficacy and safety of tap water iontophoresis is well documented, but its mechanism of action remains unknown. The most widely accepted hypothesis is that sweating is inhibited by mechanical blockage of the sweat ducts at the stratum corneum level, the depth and severity of the damage being dose-related.[23] Stripping off the stratum corneum relieves the blockage and restores sweating.[24] More recent work by Hill et al casts doubt on this theory.[25] They examined, by light and electron microscopy, sweat glands from the palm of a patient with hyperhidrosis before and after treatment and found no changes.[25]
Anesthesia Otolaryngologists have used iontophoresis of local anesthetics for anesthesia of the middle ear, and dentists, for anesthesia of the oral mucosa. [26],[27] Anesthesia of the skin can be achieved with the use of a variety of positive and negative controls, including iontophoresis of epinephrine and lidocaine separately, and topical administration of lidocaine and epinephrine.[28] Skin anesthesia is best obtained with solutions containing 1% and 4% lidocaine and between 1/10,000 and 1/50,000 epinephrine. Iontophoresis of anesthetics may be useful especially for pediatric patients.
Miscellaneous uses
Hyperkeratosis with fissuring of palms and soles: Iontophoresis with 5-10% aqueous solution of sodium salicylate showed improvement within a period of 3-4 weeks (6-8 sittings of 10-15 minutes each).[29]
Vitiligo: In an uncontrolled study, iontophoresis with meladine solution 1% in patients with vitiligo showed marked repigmentation.[30]
Scleroderma: In one trial, iontophoresis with hyaluronidase led to increased skin softness and flexibility of tissues and decreased cold sensitivity.[31] On termination of therapy, cold sensitivity returned in a week but the improvement in skin softness and flexibility persisted for three months.
Lymphedema: Iontophoresis with hyaluronidase has also been successfully used in the treatment of lymphedema of the limbs.[32]
Patch testing: Wahlberg reported encouraging results with the use of iontophoresis as a complement to ordinary patch testing in the investigation of obscure cases of contact eczema.[33] With iontophoresis, the test substances are administered rapidly and they migrate through the epidermis down into the dermis. Additionally, the disadvantages of the traditional patch test procedure, such as prolonged wearing of the test strips, are eliminated.[33]
Sweat test: Iontophoresis was also used for the diagnosis of cystic fibrosis by the sweat test. Iontophoresis with pilocarpine causes rapid sweating for minutes.[34]
Other applications of iontophoresis include introduction of "artificial skin pigment" (iron oxide and titanium oxide) into the skin,[35] iodine iontophoresis to reduce scar tissue,[36] and administration of antibiotics (penicillin) in burn patients.[37]
Conclusions
Iontophoresis has been explored for many dermatologic and other medical conditions with reports of considerable success. In many conditions, however, these explorations have been limited to a single clinical trial. More rigorous studies are needed to investigate the applications of this mode of therapy.
| 1. |
Kassan DG, Lynch AN, Stiller MJ. Physical enhancement of dermatologic drug delivery. Iontophoresis and phonophoresis. J Am Acad Derm 1996;34:656-66.
[Google Scholar]
|
| 2. |
Crumay MH. Direct iontophoresis and Galvanic surgery. In : Physical Modalities in Dermatologic Therapy 1st edn. Goldsmith H editor. New York: Springer Verlag; 1978. p. 190-6.
[Google Scholar]
|
| 3. |
Peter ME, Jui-Chen T, Gopinathan KM. Skin barrier and percutaneous drug delivery. In : Dermatology. Bolognia JL, Jorrizo JL, Rapini RP, editors. London: Mosby; 2003. p. 1969-78.
[Google Scholar]
|
| 4. |
Pikal MJ. The role of electroosmotic flow in transdermal Iontophoresis. Adv Drug Deliv Rev 2001;46:281-305.
[Google Scholar]
|
| 5. |
Rao G, Guy RH, Glikfeld P, LaCourse, Tamada J. Leung. Reverse iontophoresis: noninvasive glucose monitoring in vivo in humans. Pharm Res 1995;12:1869-73.
[Google Scholar]
|
| 6. |
Zhu H, Li SK, Peck KD, Miller DJ, Higuchi WI. Improvement on conventional constant current DC Iontophoresis: Study using constant conductance AC Iontophoresis. J Control Release 2002;82:249-61.
[Google Scholar]
|
| 7. |
Papa CM, Kligman AM. Mechanism of eccrine anhidrotic. J Invest Dermatol 1966;47:1-9.
[Google Scholar]
|
| 8. |
Abramson DI, Tuck S, Chu LS, Buso E. Physiologic and clinical basis for histamine by ion transfer. Arch Phys Med Rehabil 1967;48:583-91.
[Google Scholar]
|
| 9. |
Cornwall MW. Zinc iontophoresis to treat ischemic skin ulcers. Phys Ther 1981;61:359-60.
[Google Scholar]
|
| 10. |
Jersild O, Plesner N. Treatment of epidermophytosis in the extremities with iontophoresis of copper. Acta Derm Venereol (Stockh) 1940;21:268-79.
[Google Scholar]
|
| 11. |
Shaffer W, Zackheim HS. Sporotrichosis. Arch Derm Syph 1947;56:244-7.
[Google Scholar]
|
| 12. |
Gordon NH, Weinstein MV. Sodium salicylate iontophoresis in the treatment of plantar warts (a case report). Phys Ther 1969;49:869-70.
[Google Scholar]
|
| 13. |
Gangarosa LP, Merchant HW, Park NH, Hill JM. Iontophoretic application of idoxuridine for recurrent herpes labialis: Report of preliminary clinical findings. Methods Find Exp Clin Pharmacol 1979;1:105-9.
[Google Scholar]
|
| 14. |
Lekas MD. Iontophoresis treatment. Otolaryngol Head Neck Surg 1977;87:292-8.
[Google Scholar]
|
| 15. |
Gangarosa LP Sr. Iontophoresis in dental practice. Chicago: Quintessence Punlishing Co. Inc; 1983. p. 40-52.
[Google Scholar]
|
| 16. |
Grice K. Hyperhidrosis and its treatment by iontophoresis. Physiother 1980;66:43-4.
[Google Scholar]
|
| 17. |
Morgan K. The technique of treating hyperhidrosis by iontophoresis. Physiother 1980;66:45.
[Google Scholar]
|
| 18. |
Levit F. Treatment of hyperhidrosis by tap water iontophoresis. Cuis 1980;26:192-4.
[Google Scholar]
|
| 19. |
Shrivastava SN, Singh G. Tap water iontophoresis for palmar hyperhidrosis. Br J Dermatol 1977;96:189-95.
[Google Scholar]
|
| 20. |
Grice K, Sattar H, Baker H. Treatment of idiopathic hyerhidrosis with iontophoresis of tap water and poldine methosulfate. Br J Dermatol 1972;86:72-8.
[Google Scholar]
|
| 21. |
Abell E, Morgan K. The treatment of idiopathic hyperhidrosis by gycopyrronium bromide and tap water iontophoresis. Br J Dermatol 1974;91-87-91.
[Google Scholar]
|
| 22. |
Gibinski K, Giec L, Zmudzinski J, Dosiak J, Waclawczyk J. Transcutaneous inhibition of sweat gland function by atropine. J Appl Physiol 1973;34:850-2.
[Google Scholar]
|
| 23. |
Papa LA. The action of antiperspirants. J Soc Cosm Chem 1966;17:789-800.
[Google Scholar]
|
| 24. |
Gordon BI, Maibach HI. Eccrine anhidrosis due to glutaraldehyde, formaldehyde and iontophoresis. J Invest Dermatol 1969;53:436-9.
[Google Scholar]
|
| 25. |
Hill AC, Baker GF, Jansen GT. Mechanism of action of iontophoresis in the treatment of palmar hyperhidrosis. Cutis 1981;28:69-72.
[Google Scholar]
|
| 26. |
Comeau M, Brummett R, Vernon J. Local anesthesia of the ear by iontophoresis. Arch Otolaryngol 1973;98:114-20.
[Google Scholar]
|
| 27. |
Gangarosa LP Sr. Iontophoresis for surface local anesthesia. J Am Dent Assoc 1974;88:125-8.
[Google Scholar]
|
| 28. |
Gangarosa LP Sr. Defining a practical solution for iontophoretic local anesthesia of skin. Methods Find Exp Clin Pharmacol 1981;3:83-94.
[Google Scholar]
|
| 29. |
Mukherjee S, Gupta AB, Malakar S, Haldar B. Iontophoretic treatment of hyperkeratosis with sodium salicylate. Indian J Dermatol Venereol Leprol 1989;55:22-4.
[Google Scholar]
|
| 30. |
Moawad MB. Treatment of vitiligo with 1% solution of the sodium salt of meladine using the iontophoresis technique. Dermatol Monatsschr 155:388-94.
[Google Scholar]
|
| 31. |
Popkin RJ. The use of hyaluronidase by iontophoresis in the treatment of generalized scleroderma. J Invest Dermatol 1951;16:97-102.
[Google Scholar]
|
| 32. |
Schwartz HS. Use of hyaluronidase by iontophoresis in treatment of lymphedema. Arch Intern Med 1955;95:662-8.
[Google Scholar]
|
| 33. |
Walberg JE. Skin clearance of iontophoretically administered chromium (51Cr) and sodium (22Na) ions in the guinea pig. Acta Derm Venerol (Stockh) 1970;50:255-62.
[Google Scholar]
|
| 34. |
Sawyer CJ, Scott AV, Summer GK. Cystic fibrosis of the pancreas. A study of sweat electrolyte levels in 36 families using pilocarpine iontophoresis. South Med J 1966;59:197-202.
[Google Scholar]
|
| 35. |
Batner HB. Cataphoresis in dermabrasion tattooing. Plast Reconstr Surg 1961;27:613-7.
[Google Scholar]
|
| 36. |
Tannenbaum M. Iodine iontophoresis in reducing scar tissue. Phys Ther 1980;60:792.
[Google Scholar]
|
| 37. |
Rapperport AS, Larson DL, Henges DF, Lynch JB, Blocker TG Jr, Lewis RS. Iontophoresis - a method of antibiotic administration in the burn patient. Plast Reconstr Surg 1965;36:547-52.
[Google Scholar]
|
Fulltext Views
17,816
PDF downloads
5,779





