Translate this page into:
Janus-kinase inhibitors in dermatology: A review of their use in psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease
Corresponding author: Dr. April W Armstrong, MD, MPH, Keck School of Medicine, University of Southern California, Los Angeles, United States. armstrongpublication@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Huang MY, Armstrong AW. Janus-kinase inhibitors in dermatology: A review of their use in psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease. Indian J Dermatol Venereol Leprol. 2024;90:30-40. doi: 10.25259/IJDVL_15_2023.
Abstract
Recent studies on molecular pathways have elucidated novel therapeutic approaches in inflammatory and autoimmune skin disorders. Specifically, the dysregulation of the Janus kinase signal transducer and activator of transcription (JAK-STAT) cascade plays a central role in the pathogenesis of many skin conditions. JAK inhibitors, with their ability to selectively target immune responses, are potential treatment options. Using the National Library of Medicine, we provide a comprehensive review of the use of United States Food and Drug Administration (FDA)-approved and emerging JAK or tyrosine kinase 2 (TYK2) inhibitors in a wide range of dermatologic conditions, including psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease. In patients with psoriasis, oral deucravacitinib (TYK2 inhibitor) has been approved as a once-daily therapy with demonstrated superiority and efficacy over apremilast and placebo and tolerable safety profiles. In patients with vitiligo, topical ruxolitinib (JAK1 inhibitor) is approved as a twice-daily treatment for repigmentation. The efficacy of several other JAK inhibitors has also been demonstrated in several clinical trials and case studies for systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease. Further investigations with long-term clinical trials are necessary to confirm their utility in treatment and safety for these diseases.
Keywords
Janus-Kinase inhibitors
JAK
tyrosine kinase 2 inhibitors
TYK2
dermatology
The Janus kinase signal transducer and activator of transcription (JAK-STAT) pathway is an intracellular signalling cascade that modulates gene expression in response to cytokines.1 The JAK family includes four members [JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2)], whereas the STAT family consists of seven protein members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6).2 JAK proteins can work in different pairings. Specifically, JAK1 can pair with JAK2 or JAK3, while JAK2 can pair with itself. Depending on the pairing, JAKs can modulate various downstream intracellular signals. After cytokines bind to and activate cytokine receptors, these receptors phosphorylate their associated JAKs, which then phosphorylate the STAT proteins. Upon phosphorylation, the STATs dimerize and translocate to the cellular nucleus where they regulate gene transcription.2, 3
Dysregulation of the JAK-STAT pathway is implicated in many autoimmune and inflammatory skin disorders.4, 5 Due to its ability to selectively target immune responses, JAK inhibitors (JAKi) have been widely explored for its efficacy.6–8 By inhibiting various JAKs, JAKi can decrease downstream signal transduction of the STATs, suppressing further immune-specific responses [Figure 1].9 In dermatology, JAKi have been approved by the US Food and Drug Administration (FDA) to treat atopic dermatitis (oral abrocitinib, oral upadacitinib and topical ruxolitinib), alopecia areata (oral baricitinib), psoriasis (oral deucravacitinib) and vitiligo (topical ruxolitinib) [Table 1].
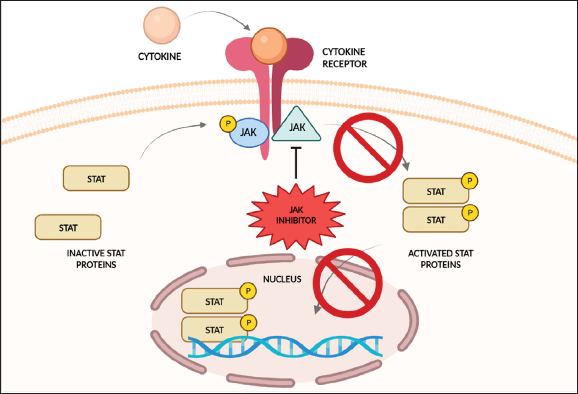
- Overview of the mechanism of action of JAK inhibitors. Binding of the JAK inhibitor to JAK proteins inhibits phosphorylation and subsequent activation of the JAK and STAT proteins. Downstream production of inflammatory cytokines is thus downregulated or inhibited.
| Inhibitor | Drug Target | Indications | Dosage | Year Approved by the FDA |
| Ruxolitinib | JAK 1/2 |
Atopic dermatitis Non-segmental vitiligo Acute graft-versus-host disease Chronic graft-versus-host disease |
Topical ruxolitinib 1.5% cream twice daily Topical ruxolitinib 1.5% cream twice daily Oral ruxolitinib 5 mg twice daily Oral ruxolitinib 10 mg twice daily |
2021 2022 2019 2021 |
| Baricitinib | JAK 1/2 | Alopecia areata |
Oral baricitinib 2 mg once daily Oral baricitinib 4 mg once daily |
2022 2022 |
| Upadacitinib | JAK1 | Atopic dermatitis |
Oral upadacitinib 15 mg once daily Oral upadacitinib 30 mg once daily |
2022 2022 |
| Abrocitinib | JAK1 | Atopic dermatitis |
Oral abrocitinib 50 mg once daily Oral abrocitinib 100 mg once daily Oral abrocitinib 200 mg once daily |
2022 2022 2022 |
| Deucravacitinib | TYK2 | Plaque psoriasis | Oral deucravacitinib 6 mg once daily | 2022 |
JAK, Janus kinase; TYK2, tyrosine kinase 2; FDA, Food and Drug Administration
Here, we review the use of FDA-approved and emerging JAK or TYK2 inhibitors in psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease. The use of JAKi for atopic dermatitis and alopecia areata will be discussed in separate articles.
Methods
The National Library of Medicine was searched via PubMed and ClinicalTrials.gov using the following search criteria: “Janus kinase inhibitor,” “JAK inhibitor,” “tyrosine kinase inhibitor,” “TYK2 inhibitor” and the name of the skin disease (e.g., “psoriasis”) from 15 September to 15 October 2022. Searches were performed without any country restrictions. Relevant case reports, case series and/or clinical studies were included, and all animal studies and pharmacologic applications were excluded.
Psoriasis
Psoriasis is an inflammatory skin disorder characterised by scaly, erythematous lesions. Around 20–30% of patients develop psoriatic arthritis (PsA) among other comorbidities.10 Its pathogenesis mainly involves abnormally upregulated Th17 responses. Central to psoriasis pathogenesis, TYK2 can pair with JAK1 or JAK2 to mediate downstream intracellular signals and is involved in the activation of myeloid dendritic cells and the activation and survival of T helper cells.11, 12 Specifically, TYK2/JAK1 pairing is important in signal transduction of type 1 interferons (IFNs) via STAT1 and STAT2.11 TYK2/JAK2 are responsible for signal transduction of IL-23 and IL-12 via STAT3 and STAT4, which are crucial for the activation and survival of Th17 and Th1 cells, respectively.12–15
There is a wide range of treatments, including topical therapies for mild replace with–psoriasis as well as oral medications and biologics for moderate-to-severe psoriasis. Oral therapies have long been important treatment options for patients; however, traditional oral agents have either limited efficacy or intolerable side effects. Thus, the search for efficacious and safe oral medications sparked the innovation and evaluation of TYK2 as a potential target.
Oral deucravacitinib is an FDA-approved, selective allosteric TYK2 inhibitor with a unique mechanism of action. It binds to the regulatory domain of TYK2, which is structurally distinct from regulatory domains of other JAKs, thus allowing for a more specific and targeted inhibition of TYK2 and reduced cross-reactivity with JAK1/2/3.16, 17 In two phase 3 trials (POETYK PSO-1 and PSO-2), oral deucravacitinib 6 mg once daily (QD) demonstrated high efficacy and tolerable safety profiles [Table 2].18–20 Specifically, in the PSO-1 trial, 58.4 and 53.6% of patients on deucravacitinib achieved ≥75% improvement in the Psoriasis Area and Severity Index and static Physician’s Global Assessment (clear or almost clear) scores compared to placebo (12.7 and 7.2%, respectively) or apremilast (35.1 and 32.1%). Therapeutic efficacy was maintained through 52 weeks of treatment. Side effects of herpes zoster and acne at low rates were observed, but the majority of cases did not lead to treatment discontinuation.18 Furthermore, VTX-958 and NDI-034858 are the emerging allosteric TYK2 inhibitors under investigation to treat psoriasis and/or PsA.21–24
| Inhibitor | Study Number | Study Design | Treatment Regimen | Results |
|
Oral Deucravacitinib |
NCT03624127 |
Phase 3, double-blind, randomised study of 666 patients (POETYK PSO-1)18 |
Deucravacitinib 6 mg QD. Apremilast 30 mg BID for initial 24 weeks. At week 24, patients who did not achieve PASI-50 were switched in a blinded fashion to deucravacitinib 6 mg QD, and patients who achieved PASI-50 continued on apremilast 30 mg BID. Placebo for initial 16 weeks, followed by deucravacitinib 6 mg QD. All treated for 52 weeks. |
At week 16, PASI-75 and sPGA (0 or 1) response rates were significantly higher in patients on deucravacitinib vs placebo or apremilast (58.4% vs 12.7% vs 35.1%; p < 0.0001) and (53.6% vs 7.2% vs 32.1%; p < 0.0001). Efficacy of deucravacitinib improved beyond week 16 and was maintained through week 52. |
| Oral Deucravacitinib | NCT03611751 | Phase 3, double-blind, randomised study of 1020 patients (POETYK PSO-2)19 |
Deucravacitinib 6 mg QD for initial 24 weeks. At week 24, patients who achieved PASI-75 were re-randomised 1:1 to placebo or deucravacitinib 6 mg QD, and those who did not achieve PASI-75 continued deucravacitinib 6 mg QD. Apremilast 30 mg BID for initial 24 weeks. At week 24, patients who did not achieve PASI-75 were switched in a blinded fashion to deucravacitinib 6 mg QD, and patients who achieved PASI-75 were continued on placebo. Placebo for initial 16 weeks, followed by deucravacitinib 6 mg QD. All treated for 52 weeks. |
At week 16, PASI-75 and sPGA (0 or 1) response rates were significantly higher in patients on deucravacitinib vs placebo or apremilast (53.0% vs 9.4% vs 39.8%; p < 0.0001) and (49.5% vs 8.6% vs 33.9; p < 0.0001). Among deucravacitinib-treated patients who achieved PASI-75 at week 24 and were randomised to continue deucravacitinib, PASI-75 responses were maintained through week 52 (80.4% [119/148]; sPGA 0 or 1, 70.3% [83/118]). |
| Oral Deucravacitinib | NCT03881059 | Phase 2, double-blind, randomised study of 203 patients20 |
Deucravacitinib 6 mg QD. Deucravacitinib 12 mg QD. Placebo QD. All treated for 16 weeks. |
At week 16, ACR-20 response was significantly higher in deucravacitinib 6 mg (52.9%, p = 0.0134) and 12 mg (62.7%, p = 0.0004) compared to placebo (31.8%). Both deucravacitinib doses resulted in significant improvements versus placebo ( p £ 0.05) from baseline in HAQ-DI and SF-36 PCS score as well as PASI-75 response. |
TYK2, tyrosine kinase 2; QOD, every other day; QD, once daily; BID, twice daily; PASI-50, ≥50% improvement from baseline in Psoriasis Area and Severity Index (PASI) scores; PASI-75, ≥ 75% improvement from baseline in Psoriasis Area and Severity Index (PASI) scores; sPGA (0 or 1), static Physician’s Global Assessment score of clear or almost clear; ACR-20; American College of Rheumatology-20; HAQ-DI, Health Assessment Questionnaire-Disability Index; SF-36 PCS, Short Form-36 (SF-36) Physical Component Summary (PCS) score.
The efficacy of tofacitinib, a JAK1/3 inhibitor, in psoriasis and nail psoriasis was also investigated [Table 3].25–29 Currently, oral tofacitinib and oral upadacitinib are approved to treat PsA only, not psoriasis.30 Other JAKi including ruxolitinib (JAK1/2 inhibitor), baricitinib (JAK1/2 inhibitor) and solcitinib (JAK1 inhibitor) have been evaluated. However, due to their underwhelming performances and adverse events (AEs), there are no current plans for further development.31–36
| Inhibitor | Study Number | Study Design | Treatment Regimen | Results |
| Oral Tofacitinib |
NCT01276639 NCT01309737 |
Phase 3, double-blind, randomised study of 901 and 960 patients (Parallel OPT Pivotal 1 and Pivotal 2 studies)25 |
Tofacitinib 5 mg BID. Tofacitinib 10 mg BID. Placebo BID. All treated for 16 weeks. |
At week 16, PASI-75 and PGA (0 or 1) response rates for both studies were significantly higher in patients on 5 mg and 10 mg compared to those on placebo (p < 0.001 in both studies for each dose). At week 16, significantly more patients on 10 mg achieved PGA (0 or 1) response ( p < 0.001) and PASI 75 ( p < 0.001) compared to group 1 in both studies. |
| Oral Tofacitinib | NCT01241591 | Phase 3, double-blind, randomised study of 1106 patients26 |
Tofacitinib 5 mg BID. Tofacitinib 10 mg BID. Etanercept 50 mg subcutaneously BIW. Placebo BID. All treated for 12 weeks |
At week 12, PASI-75 and PGA (0 or 1) response rates were significantly higher in both tofacitinib groups and etanercept group compared to placebo ( p < 0.0001 for all 3 groups). Tofacitinib 10 mg BID was noninferior to etanercept and superior to placebo. |
| Oral Tofacitinib | NCT01186744 | Phase 3, double-blind, randomised study with withdrawal and retreatment of 666 patients27 |
Initial Phase (24 weeks): Tofacitinib 5 mg BID. Tofacitinib 10 mg BID. Withdrawal Phase (Up to 16 weeks): Those who achieved PASI-75 response and a PGA (0 or 1) response in the initial phase were randomised to placebo BID or their previous dose of tofacitinib (5 mg or 10 mg BID). Retreatment Phase (Ended at 56 weeks): Patients were retreated with the same dose of tofacitinib (5 or 10 mg) that they received in the initial phase. |
Initial Phase: At week 24, 33.5% (5 mg) and 55.2% (10 mg) of patients achieved both PASI-75 and a PGA (0 or 1) response and continued to the withdrawal phase. Withdrawal Phase: Significantly greater proportions of patients on tofacitinib maintained a PASI-75 response compared to those who switched to placebo (5 mg vs placebo; p = 0.008); (10 mg vs placebo; p < 0.0001). At week 16, 92.3% (5 mg) and 93.0% (10 mg) had no relapse, compared to 32.8% (placebo, 5 mg in the initial phase) and 42.9% (placebo, 10 mg in the initial phase). Retreatment Phase: After 16 weeks of retreatment, among patients treated with placebo in the withdrawal phase, 48.0/52.0% (5 mg retreatment) and 72.5/64.2% (10 mg retreatment) regained or maintained a PASI-75 or PGA response. Among patients who relapsed on placebo in the withdrawal phase, 36.8% (5 mg) and 61.0% (10 mg) achieved a PASI-75 response, while 44.8% (5 mg) and 57.1% (10 mg) of those who lost a PGA response achieved a PGA (0 or 1) response upon retreatment. |
| Oral Tofacitinib | NCT01815424 | Phase 3, double-blind, randomised study of 266 Asian patients28 |
Initial Phase (16 weeks): Tofacitinib 5 mg BID. Tofacitinib 10 mg BID. Placebo BID. Active Treatment Phase (52 weeks): Those who received tofacitinib 5 mg or 10 mg BID in the initial phase continued on the same dose. Those who were previously on placebo were switched to either 5 mg or 10 mg BID. Placebo BID. |
At Week 16, significantly greater proportions of patients who received tofacitinib 5 mg BID (52.3%; 54.6%) and 10 mg BID (75.6%; 81.1%) achieved PASI-75 and PGA (0 or 1) response compared to placebo (19.3%; 12.5%; all p < 0.0001). Both PGA (0 or 1) and PASI-75 responses were maintained from Week 16 through Week 52 with tofacitinib 5 mg and 10 mg BID. |
| Oral Tofacitinib |
NCT01276639 NCT01309737 |
Two Phase 3, double-blind, randomised, parallel studies of 1,196 patients (pooled from parallel OPT Pivotal 1 and Pivotal 2 studies)29 |
Tofacitinib 5 mg BID. Tofacitinib 10 mg BID. Placebo BID. All treated for 16 weeks. |
At Week 16, significantly greater proportions of patients with nail psoriasis receiving tofacitinib 5 mg BID and 10 mg BID versus placebo achieved NAPSI-50 (32.8%; 44.2% vs 12.0%; p < 0.05), NAPSI-75 (16.9%; 28.1% vs 6.8%; p < 0.05), and NAPSI-100 (10.3%; 18.2% vs 5.1%; p < 0.05). Efficacy of tofacitinib was maintained through week 52. |
JAK, Janus kinase; BID, twice daily; PASI-75, ≥ 75% improvement from baseline in Psoriasis Area and Severity Index (PASI) scores; OPT, oral-treatment psoriasis trial; PGA (0 or 1), Physician’s Global Assessment score of clear or almost clear; BIW, twice a week; NAPSI-50, ≥ 50% improvement from baseline in Nail Psoriasis Severity Index (NAPSI) scores; NAPSI-75, ≥ 75% improvement from baseline in Nail Psoriasis Severity Index (NAPSI) scores; NAPSI-100, 100% improvement from baseline in Nail Psoriasis Severity Index (NAPSI) scores.
Vitiligo
Vitiligo is an autoimmune disorder characterised by depigmented macules or patches. Its pathogenesis involves self-reactive CD8+ T cells and destruction of melanocytes.37 IFN-γ, which is a key cytokine produced by CD8+ T cells, activates JAK1 and JAK2, leading to the phosphorylation of STAT1 and STAT2 and further recruitment of CD8+ T cells.37, 38 Thus, JAKi have been extensively studied for their therapeutic potentials [Table 3].39–42
Ruxolitinib 1.5% cream is a JAK1/2 inhibitor approved to treat depigmentation in patients with non-segmental vitiligo.43 In two phase 3 TRuE-V1 and TruE-V2 studies, 30.7% of patients who applied ruxolitinib 1.5% cream BID achieved ≥75% improvement in the facial Vitiligo Area Scoring Index compared to 9.9% of patients on vehicle cream BID at week 24. Common AEs included application-site reactions.39, 40
Notably, sun-exposed areas, such as the face, demonstrated preferential repigmentation in several case studies.44–48 A meta-analysis evaluating 45 patients found that 88.9% of patients on both JAKi and phototherapy achieved better repigmentation results compared to 11.1% of patients on JAKi monotherapy.49 Thus, concomitant treatments of JAKi (to suppress immune response) and light therapy (to stimulate melanocyte regeneration) may be necessary to achieve maximum repigmentation.49 The efficacy of other JAKi has also been evaluated in several case studies [Table 4].50–54
| Inhibitor | Drug Target |
Study Number |
Study Design | Treatment Regimen | Results |
| Topical Ruxolitinib | JAK 1/2 |
NCT04052425 NCT04057573 |
Two Phase 3, double-blind, randomised, parallel studies of 330 and 334 patient (TRuE-V139 and TruE-V240) |
Ruxolitinib 1.5% cream BID. Placebo BID. All treated for 24 weeks (primary), 52 weeks (secondary). |
At week 24, significantly greater proportions of patients applying ruxolitinib cream BID achieved F-VASI75 compared to those on vehicle BID (30.7% vs 9.9%; p < .0001). At week 52, approximately 50% of patients showed ≥75% improvement in F-VASI compared to 30% of patients at Week 24. At week 52, approximately 75% of patients showed ≥50% improvement in F-VASI compared to 51% of patients at Week 24. At week 52, approximately 30% of patients showed ≥90% improvement in F-VASI compared to 15% of patients at Week 24. |
| Topical Ruxolitinib | JAK 1/2 |
NCT03099304 |
Phase 2, double-blind, randomised study of 157 patients41 |
Ruxolitinib 1.5% cream BID. Ruxolitinib 1.5% cream QD. Ruxolitinib 0.5% cream QD. Ruxolitinib 0.15% cream QD. Matched placebo. All treated for 24 Weeks |
At week 24, patients on ruxolitinib cream 1.5% BID and 1.5% QD demonstrated a ≥50% improvement from baseline F-VASI (1.5% cream BID, OR 24.7; 95% CI, 3.3-1121.4; p = .0001); (1.5% cream QD, OR 28.5; 95% CI, 3.7-1305.2; p = .0001) compared to patients on placebo. |
|
Topical Ifidancitinib (ATI-50002) |
JAK 1/3 |
NCT03468855 | Phase 2, open-label, nonrandomised, single-group study of 34 patients42 | Ifidancitinib 0.46% solution BID for 24 weeks. | At week 24, there was an improvement in mean F-VASI: -0.067 ( SD : 0.2411). The VNS scale change was 2.2 ( SD : 0.66). |
| Topical Tofacitinib | JAK 1/3 | - | Nonrandomised cohort study of 16 patients50 | Tofacitinib 2% cream BID with concomitant TCS, TCI, supplements ( Polypodium leucotomos and Ginkgo biloba ), NB-UVB, PUVA, or excimer laser. | 4 patients showed ≥90% repigmentation, 5 patients showed 25–75% repigmentation, and 4 patients showed 5-15% repigmentation. Facial vitiligo improved more than non-facial vitiligo ( p = 0.0216). Patients with Fitzpatrick skin types IV–VI improved more than those with lighter skin types ( p = 0.3434). |
| Topical Tofacitinib | JAK 1/3 | - | Nonrandomised cohort study of 11 patients51 | Tofacitinib 2% cream BID with NB-UVB three times weekly for 8-16 weeks | There was a mean improvement of 70% (50–87%) in F-VASI score in all 11 patients. |
| Topical Tofacitinib | JAK 1/3 | - |
Case report of 1 patient52 |
Tofacitinib 2% cream BID with NB-UVB three times weekly for 36 weeks | At Week 36, Patient showed significant repigmentation of the face, especially around the perioral and periorbital regions. |
| Topical Delgocitinib |
JAK 1/2/3; TYK2 |
- |
Case report of 2 patients47 |
Patient 1: Delgocitinib cream BID for 8 weeks Patient 2: Delgocitinib cream BID for 12 weeks |
At week 8, patient 1 showed significant repigmentation of the neck. At week 12, Patient 2 did not show repigmentation of left elbow. |
| Oral Tofacitinib | JAK 1/3 | - |
Case report of 1 patient53 |
Patient 1: Tofacitinib 5 mg every other day, followed by 5 mg QD for 20 weeks | At week 20, there was nearly complete repigmentation of the forehead and hands. Approximately 5% of the total body surface area remained depigmented. |
| Oral Tofacitinib | JAK 1/3 | - | Retrospective case series of 10 patients44 | Tofacitinib 5–10 mg QD or BID for an average of 36 weeks | There was a mean decrease of 5.4% total body surface area in 5/10 patients, and repigmentation occurred in sun-exposed areas in 3 of them. The other 5 patients did not show any repigmentation. |
| Oral Tofacitinib | JAK 1/3 | - | Case report of 2 patients45 |
Patient 1: Tofacitinib 5 mg BID and NV-UVB twice weekly for 12 weeks Patient 2: Tofacitinib 5 mg BID and NV-UVB two or three times weekly for 12 weeks (primary), 36 weeks (secondary). |
At week 12, patient 1 showed nearly complete repigmentation of the face and ≥75% repigmentation of the neck, chest, and upper and lower extremities. At week 12, patient 2 showed 50% repigmentation of the face. At week 36, there was 75% repigmentation of the face. No repigmentation observed at other areas. |
| Oral Baricitinib | JAK ½ | - | Case report of 1 patient54 | Baricitinib 4 mg QD for 32 weeks. | At week 32, there was nearly complete repigmentation of the hands and forearms. |
BID, twice daily; JAK, Janus kinase; CI, confidence interval; VASI , Vitiligo Area Scoring Index; F-VASI, facial Vitiligo Area Scoring Index; F-VASI75, ≥ 75% improvement in the facial Vitiligo Area Scoring Index; QD, once daily; OR, odds ratio; VNS, Vitiligo Noticeability Scale; SD, standard deviation; TCS, topical corticosteroid; TCI, topical calcineurin inhibitor; NB-UVB, narrow-band ultraviolet; PUVA, psoralen-ultraviolet A; QD, once daily; F-VASI, facial Vitiligo Area Scoring Index; TYK2, tyrosine kinase 2.
Systemic Lupus Erythematosus (SLE)
SLE is an autoimmune disorder with variable clinical presentations. Approximately 80% of patients develop cutaneous symptoms.55 Many cytokines involved in SLE pathogenesis, including type 1 IFNs and IL-23, signal through the JAK-STAT pathway. By activating JAK1 and TYK2, type 1 IFNs stimulate dendritic cells via STAT1 signalling, thereby leading to the development of autoantibodies by B cells.56, 57 Current treatments rely on corticosteroids, nonsteroidal immunosuppressants and two FDA-approved biologics (belimumab and anifrolumab). However, additional therapies are needed.58
The efficacy of baricitinib was evaluated in two parallel phase 3 studies, SLE-BRAVE-I and SLE-BRAVE-II [Table 5].59–61 In SLE-BRAVE-I, 56.7% of patients on oral baricitinib 4 mg QD achieved SLE Responder Index-4 (SRI-4) compared to 45.9% of patients on placebo at week 52. However, no significant results were found in SLE-BRAVE-II.59 Therefore, further analyses are needed to investigate the efficacy of baricitinib for SLE.
| Inhibitor | Drug Target | Study Number | Study Design | Treatment Regimen | Results |
| Oral Baricitinib | JAK 1/2 |
NCT03616912 NCT03616964 |
Two Phase 3, double-blind, randomised, parallel studies of 760 and 775 patients (SLE-BRAVE-I and SLE-BRAVE-II)59 |
Baricitinib 2 mg QD. Baricitinib 4 mg QD. Placebo QD. All treated for 52 weeks. |
In SLE-BRAVE-I, significantly greater proportions of patients who received baricitinib 4 mg achieved SRI-4 responses compared to placebo (56.7% vs 45.9%, p = 0.016) at week 52. No significant differences were seen in patients who received baricitinib 2 mg (49.8%) compared to placebo (45.9%). In SLE-BRAVE-II, no differences were seen between patients who received baricitinib 4 mg, 2 mg, and placebo (47.1%, 46.3%, and 45.6%, respectively). |
| Oral Baricitinib | JAK 1/2 | NCT02708095 | Phase 2, double-blind, randomised study of 314 patients60 |
Baricitinib 2 mg QD. Baricitinib 4 mg QD. Placebo QD. All treated for 24 weeks. |
At week 24, significantly greater proportions of patients on baricitinib 4 mg achieved SLEDAI-2K (OR 1.8; 95% CI, 1.0-3.3; p = 0.0414) and SRI-4 (OR 2.0; 95% CI 1.2-3.6; p = 0.0151) compared to placebo. No significant differences were found between patients who received baricitinib 2 mg vs placebo (OR 1.3, 0.7–2.3; p = 0.39; OR 1.3, 95% CI, 0.7–2.2; p = 0.44). |
| Oral Deucravacitinib (BMS-986165) | TYK 2 | NCT03252587 |
Phase 2, double-blind, randomised study of 314 patients (PAISLEY)61 |
Deucravacitinib 3 mg BID. Deucravacitinib 6 mg BID. Deucravacitinib 12 mg QD. Placebo BID. All treated for 32 weeks. |
At week 32, significantly greater proportions of patients in the 3 mg and 6 mg group achieved SRI-4 compared to placebo (deucravacitinib 3 mg BID: 58.2%, p = 0.0006; deucravacitinib 6 mg BID: 49.5%, p = 0.0210; placebo: 34.4%). The 12 mg group had numerically higher SRI-4 response rates than placebo, but differences were nonsignificant. The efficacy of deucravacitinib was maintained through 48 weeks. |
QD, once daily; JAK, Janus kinase, SRI-4, Systemic Lupus Erythematosus Responder Index-4; SLEDAI-2K, resolution of arthritis or rash; OR, odds ratios; CI, confidence interval; BID, twice daily, TYK2. tyrosine kinase 2
Because type 1 IFNs signal through TYK2, TYK2 inhibitors like deucravacitinib are potential agents. In a phase 2 study, significantly greater proportions of patients on oral deucravacitinib 3 mg BID and 6 mg BID achieved SRI-4 responses than those on placebo [Table 6].61, 62 Additionally, brepocitinib (TYK2/JAK1 inhibitor) and elsubrutinib (Bruton’s tyrosine kinase inhibitor) are currently under investigation.63, 64
| Inhibitor | Drug Target | Disease Treated | Adverse Effects |
| Topical Ruxolitinib | JAK1/2 | Vitiligo | Application site acne, application site pruritus, nasopharyngitis, headache, urinary tract infections, application erythema and pyrexia. |
| Topical Tofacitinib | JAK1/3 | Vitiligo | Acne, skin contour change, erythema and acne. |
| Topical Ifidancitinib (ATI-50002) | JAK1/3 | Vitiligo | URTI, application site dryness, application site rash. |
| Topical Delgocitinib | JAK1/2/3; TYK2 | Vitiligo | No serious adverse event reported. |
| Oral Baricitinib | JAK1/2 | Vitiligo, Systematic lupus erythematosus | Serious infections and deep-vein thrombosis |
| Oral Tofacitinib | JAK1/3 | Psoriasis, nail psoriasis, vitiligo | URTI, nasopharyngitis, increased blood creatine phosphokinase and cholesterol, hyperlipidemia, headache, herpes zoster infection, folliculitis and viral gastroenteritis. |
| Oral Deucravacitinib | TYK2 | Psoriasis, psoriatic arthritis, systematic lupus erythematosus | URTI, nasopharyngitis, headache, dermatitis acneiform, acne, diarrhoea, increased blood creatine phosphokinase, herpes simplex, mouth ulcers, folliculitis and urinary tract infections. |
JAK, Janus kinase; URTI, upper respiratory tract infection; TYK2, tyrosine kinase 2.
Hidradenitis Suppurativa (HS)
HS is an inflammatory disorder involving an occlusion of hair follicles and bacterial accumulation.65 Patients develop painful abscesses, nodules or fistulas in the skinfolds of axillary, inguinal and gluteal regions.65 Current first-line treatments rely on antibiotics and hormonal therapies. Although adalimumab (TNF-α inhibitor) was approved to treat HS, it has varying responses.66 Thus, there remains a need for therapeutic alternatives.
Recent studies revealed that HS pathogenesis is primarily driven by TNF-α and IFN-γ via STAT1.67–69 Thus, JAKi may be potentially useful in treatment. There are reports of HS successfully treated with oral tofacitinib 5 mg BID.70 Additionally, the efficacy of JAK1 inhibitor, INCB054707, was investigated in a phase 2 randomised trial. Seventeen patients (65%) who received INCB054707 30 mg, 60 mg, or 90 mg QD achieved HS Clinical Response compared to 4 patients (57%) on placebo.71 Common AEs included upper respiratory tract infections (URTIs). Several JAKi are also being evaluated, including ruxolitinib, upadacitinib (JAK1 inhibitor), PF-0682664 (TYK2 inhibitor) and PF-06700841 (TYK2/JAK1 inhibitor).72–76
Other Dermatologic Disorders
Dermatomyositis
Dermatomyositis is an autoimmune disorder characterised by a range of muscular and cutaneous symptoms. Multiple autoantibodies are associated with the disease, and depending on organ involvement, dermatomyositis may affect the lungs, esophagus or heart.77, 78 Previous studies suggested that dermatomyositis is primarily driven by type I and II IFNs via STAT1.79 Although current treatments rely on corticosteroids and nonsteroidal immunosuppressants, relapses are common and difficult to manage.80
There have been successful attempts at treating refractory dermatomyositis with oral tofacitinib and oral ruxolitinib.81–84 In an open-label study consisting of ten patients with refractory dermatomyositis, all patients on tofacitinib 11 mg QD achieved significant improvements in lesion severity after 12 weeks.81 Additionally, oral baricitinib and oral brepocitinib are under investigation in ongoing phase 2 and 3 trials.85–88
Lichen Planus (LP) and Lichen Planopilaris (LPP)
LP is an inflammatory skin and mucous disorder presenting with polygonal papules and plaques. There are several morphological variants, with nails and oral cavity commonly affected.89 Recent studies revealed that IFN-γ is highly expressed in LP skin samples, which suggests that JAKi have therapeutic potential.90 Three patients with recalcitrant LP were treated with oral tofacitinib 5 mg BID. Two patients achieved remission, and the third had substantial improvements on tofacitinib, methotrexate and prednisone. No AEs were reported.90 In a phase 2 pilot study, 12 patients receiving topical ruxolitinib 1.5% cream BID for 8 weeks showed significant improvements in lesion severity and count.91
Patients with lichen planopilaris (LPP), which is a morphological variant of LP classified as a primary lymphocytic cicatricial alopecia, also responded well to oral tofacitinib. In a case series of ten LPP patients receiving tofacitinib (as monotherapy or adjunctive therapy), there was a significant improvement.92 Thus, JAKi have the potential to be alternative treatments for this condition.
Sarcoidosis
Sarcoidosis is an inflammatory disease classically associated with non-caseating granulomas. Cutaneous symptoms occur in one-third of patients and can present variably as plaques, nodules or lupus pernio.93, 94 Sarcoidal granulomas are composed of macrophages and T cells that secrete IL-6 and IFN-γ. STAT1 is primarily activated in granuloma macrophages, whereas STAT3 is activated in between lymphocytic granulomas.95 There are reports of cutaneous sarcoidosis successfully treated with oral tofacitinib, ruxolitinib and baricitinib.96–102 Likewise, molecular analyses demonstrated that oral tofacitinib reduced levels of proinflammatory cytokines in patients with cutaneous sarcoidosis, indicating that JAKi are potential treatments.97
In an open-label trial, ten patients with cutaneous sarcoidosis received oral tofacitinib 5 mg BID. After 6 months of treatment, all patients experienced improvement in their skin, with six of them showing complete remission. Those with concurrent pulmonary and myocardial sarcoidosis also demonstrated improvements. No significant AEs were observed.103 These results are promising and support further investigation of JAKi as possible treatment modalities for sarcoidosis.
Graft-versus-Host Disease (GvHD)
GvHD is a serious complication following allogeneic stem-cell transplantation. Donor T cells (graft) recognise the recipient antigens (host) as “foreign,” thereby initiating a graft-versus-host reaction.104 Acute GvHD (aGvHD) presents as maculopapular rash, and chronic GvHD (cGvHD) can present variably as lesions resembling LP or scleroderma.105 IFN-γ and IFN-α/β are key cytokines that signal through JAK1/JAK2 and JAK1/TYK2 via STAT1, respectively.106
Oral ruxolitinib is a JAK1/2 inhibitor approved to treat aGvHD and cGvHD. In a phase 3 trial (REACH-2), patients with aGvHD receiving oral ruxolitinib 10 mg BID achieved a higher overall response rate (ORR) and longer median survival than the controls, who were on best available therapy.107 Overall response was higher in the ruxolitinib group than in the control group (62% [96 patients] vs. 39% [61]; OR, 2.64; 95% confidence interval [CI], 1.65–4.22; P < 0.001). In another phase 3 REACH-3 trial, patients with steroid-refractory cGvHD who received ruxolitinib 10 mg BID had a higher ORR than the controls. Common AEs in both studies included thrombocytopenia and anemia.108
In a single-arm, phase 1/2 study assessing the efficacy of oral baricitinib in patients with refractory cGvHD, preliminary analyses revealed that patients on baricitinib had a high ORR at 6-months was 63% (95% CI 47–87%) in 7 of 8 patients at 12-months. Common AEs included URTIs and neutropenia.109 Several other JAKi are also under investigation for GvHD.110,111
Conclusion
JAKi, with its ability to inhibit members of the JAK proteins and suppress downstream intracellular signals, have emerged as an exciting, new class of small-molecule therapy with growing indications in dermatology. Compared to other immune-targeted injectable therapies such as biologics, JAKi can be given either orally or applied topically, which makes them increasingly accepted as convenient therapeutic options. As discussed, JAKi offer promising results for a number of dermatologic diseases. As we continue to understand the roles of JAK-STAT-signalling pathway in these diseases, further studies are needed to explore which cytokines and JAKs can be selectively targeted in order to reduce cross-reactivity. Thus, the specificity of JAKi remains as an area of constant investigation and innovation.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Author MYH has no conflicts of interest to declare. AWA has served as a research investigator, scientific advisor, and/or speaker to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, BI, BMS, EPI, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed.
References
- JAK/STAT pathway modulation: Does it work in dermatology? Dermatol Ther 2019;32:e12903.
- Targeting JAK/STAT signaling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol. 2017;47:1096-107.
- [CrossRef] [PubMed] [Google Scholar]
- JAK inhibitors in dermatology: The promise of a new drug class. J Am Acad Dermatol. 2017;76:736-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-8.
- [CrossRef] [PubMed] [Google Scholar]
- JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;11:e0164080.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Janus kinase inhibitors in dermatology: A systematic review. J Am Acad Dermatol. 2017;76:745-53. e19
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinase inhibitors in dermatology: Part I. A comprehensive review. J Am Acad Dermatol. 2022;86:406-13.
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinase inhibitors in dermatology: Part II. A comprehensive review. J Am Acad Dermatol. 2022;86:414-22.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative efficacy and safety of abrocitinib, baricitinib, and upadacitinib for moderate-to-severe atopic dermatitis: A network meta-analysis. Dermatol Ther. 2022;35:e15636.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The JAK-STAT pathway at twenty. Immunity. 2012;36:503-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21:425-34.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mechanisms of disease: psoriasis. The New England Journal of Medicine. 2009;361:444-509.
- [CrossRef] [PubMed] [Google Scholar]
- Cracking the cytokine code in psoriasis. Nature Medicine. 2007;13:242-244.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis: A STAT3-Centric View. Int J Mol Sci. 2018;19:171.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- TYK2 in immune responses and treatment of psoriasis. J Inflamm Res. 2022;15:5373-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- JAK inhibitors for treatment of psoriasis: Focus on selective TYK2 inhibitors. Drugs. 2020;80:341-52.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol 2022:S0190-962202256-3.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 POETYK PSO-2 trial. J Am Acad Dermatol 2022:S0190-962202643-3.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81:815-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ventyx biosciences announces positive topline phase 1 data for its selective allosteric TYK2 inhibitor VTX958. Ventyx Biosciences. Published August 15, 2022. Accessed October 1, 2022. https://ir.ventyxbio.com/news-releases/news-release-details/ventyx-biosciences-announces-positive-topline-phase-1-data-its-0
- Nimbus therapeutics announces expansion of oral allosteric TYK2 inhibitor program and provides additional business updates. Nimbus therapeutics. Published January 6, 2022. Accessed October 1, 2022. https://www.nimbustx.com/2022/01/06/nimbus-therapeutics-announces-expansion-of-oral-allosteric-tyk2-inhibitor-program-and-provides-additional-business-updates/
- Study of NDI-034858 in subjects with moderate to severe plaque psoriasis. Identifier NCT04999839.
- A study to evaluate the efficacy, safety, and tolerability of NDI-034858 in subjects with active psoriatic arthritis. Identifier NCT05153148.
- Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949-61.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomized non-inferiority trial. Lancet. 2015;386:552-61.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2015;172:1395-406.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: A Phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88:36-45.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of tofacitinib for the treatment of nail psoriasis: Two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77:79-87. e1
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib in the management of active psoriatic arthritis: patient selection and perspectives. Psoriasis (Auckl). 2019;9:97-107.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-64.
- [CrossRef] [PubMed] [Google Scholar]
- open label, safety and efficacy study of topical investigational drug to treat patients with psoriasis. Identifier NCT00617994.
- Tofacitinib and other kinase inhibitors in the treatment of psoriasis. Actas Dermo-Sifiliográficas. 2013;104:304-310.
- [PubMed] [Google Scholar]
- A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2016;174:1266-76.
- [CrossRef] [PubMed] [Google Scholar]
- European Medicines Agency assessment report: Baricitinib. Published 2016. Accessed Oct 2, 2022. https://www.ema.europa.eu/en/documents/assessment-report/olumiant-epar-public-asses sment-report_en.pdf.
- Investigation of selective JAK1 inhibitor GSK2586184 for the treatment of psoriasis in a randomized placebo-controlled phase IIa study. Br J Dermatol. 2016;174:985-95.
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinase inhibitors in the treatment of vitiligo: A review. Front Immunol. 2021;12:790125.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). Identifier NCT04052425.
- Topical ruxolitinib evaluation in vitiligo study 2 (TRuE-V2). Identifier NCT04057573.
- Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-20.
- [CrossRef] [PubMed] [Google Scholar]
- A study of ATI-50002 topical solution for the treatment of vitiligo. Identifier NCT03468855.
- FDA approves Ruxolitinib (Opzelura) for Vitiligo Therapy: A breakthrough in the field of dermatology. Ann Med Surg (Lond). 2022;81:104499.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682. e1
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rapid repigmentation of vitiligo using tofacitinib plus low-dose, narrowband UV-B phototherapy. JAMA Dermatol. 2018;154:370-1.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of light in the treatment of vitiligo with JAK-inhibitors. J Dermatolog Treat. 2018;29:98-9.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of vitiligo vulgaris treated with topical Janus kinase inhibitor delgocitinib. Australas J Dermatol. 2021;62:433-4.
- [CrossRef] [PubMed] [Google Scholar]
- A literature review investigating the use of topical janus kinase inhibitors for the treatment of vitiligo. J Clin Aesthet Dermatol. 2022;15:20-5.
- [CrossRef] [PubMed] [Google Scholar]
- Repigmentation in vitiligo using janus kinase (JAK) inhibitors with phototherapy: systematic review and Meta-analysis. J Dermatolog Treat. 2022;33:173-7.
- [CrossRef] [PubMed] [Google Scholar]
- Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br J Dermatol. 2020;182:1047-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-8.
- [CrossRef] [PubMed] [Google Scholar]
- Topical tofacitinib: A janus kinase inhibitor for the treatment of vitiligo in an adolescent patient. Case Rep Dermatol. 2021;13:190-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tofacitinib citrate for the treatment of vitiligo: A pathogenesis-directed therapy. JAMA Dermatol. 2015;151:1110-2.
- [CrossRef] [PubMed] [Google Scholar]
- Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous lupus and the cutaneous lupus erythematosus disease area and severity index instrument. Rheum Dis Clin North Am. 2010;36:33-vii.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819-31.
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18:133-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- POS0190 Efficacy and safety of baricitinib in patients with systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, parallel-group, phase 3 studies. Annals of the Rheumatic Diseases 2022:81:327-8.
- [Google Scholar]
- Baricitinib for systemic lupus erythematosus: a double-blind, randomized, placebo-controlled, phase 2 trial [published correction appears in Lancet. 2018 Aug 11;392:476] Lancet. ;392:222-31.
- [CrossRef] [PubMed] [Google Scholar]
- LB0004 Efficacy and safety of deucravacitinib, an oral, selective, allosteric TYK2 inhibitor, in patients with active systemic lupus erythematosus: A Phase 2, randomized, double-blind, placebo-controlled study. Annals of the Rheumatic Diseases. 2022;81:209.
- [Google Scholar]
- An investigational study to evaluate BMS-986165 in participants with systemic lupus erythematosus. Identifier NCT03252587.
- A dose-ranging study to evaluate efficacy and safety of PF-06700841 in systemic lupus erythematosus (SLE). Identifier NCT03845517.
- A study to investigate the safety and efficacy of elsubrutinib and upadacitinib given alone or in combination in participants with moderately to severely active systemic lupus erythematosus (SLE) (SLEek). Identifier NCT03978520.
- Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- achieving hidradenitis suppurativa response score is associated with significant improvement in clinical and patient-reported outcomes: Post hoc analysis of pooled data from PIONEER I and II. Acta Derm Venereol. 2018;98:932-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J Invest Dermatol. 2016;136:1768-80.
- [CrossRef] [PubMed] [Google Scholar]
- Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-39.
- [CrossRef] [PubMed] [Google Scholar]
- Stress signalling and STAT1 activation characterize the keratinocytic gene expression pattern in Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2022;36:2488-98.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib shows benefit in conjunction with other therapies in recalcitrant hidradenitis suppurativa patients. JAAD Case Rep. 2020;6:99-102.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: results from two phase II studies. Br J Dermatol. 2022;186:803-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Janus kinase inhibitors for hidradenitis suppurativa: expanding the therapeutic toolbox. Br J Dermatol. 2022;186:768-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical implementation of biologics and small molecules in the treatment of hidradenitis suppurativa. Drugs. 2021;81:1397-410.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Topical Ruxolitinib 1.5% for hidradenitis suppurativa treatment. Identifier NCT04414514.
- A study of oral upadacitinib tablet compared to placebo in adult participants with moderate to severe hidradenitis suppurativa to assess change in disease symptoms. Identifier NCT04430855.
- A study to evaluate the safety and efficacy of PF-06650833, PF-06700841, and PF 06826647 in adults with hidradenitis suppurativa. Identifier NCT04092452.
- Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35:438-44.
- [PubMed] [Google Scholar]
- Molecular profiles of inflammatory myopathies. Neurology. 2002;59:1170-82.
- [CrossRef] [PubMed] [Google Scholar]
- JAK-inhibitors for dermatomyositis: A concise literature review. Dermatol Ther. 2021;34:e14939.
- [CrossRef] [PubMed] [Google Scholar]
- Study of tofacitinib in refractory dermatomyositis: An open-label pilot study of ten patients. Arthritis Rheumatol. 2021;73:858-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A case of refractory dermatomyositis responsive to tofacitinib. Semin Arthritis Rheum. 2017;46:e19.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib citrate for refractory cutaneous dermatomyositis: An alternative treatment. JAMA Dermatol. 2016;152:944-5.
- [CrossRef] [PubMed] [Google Scholar]
- Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med. 2014;371:2537-8.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of JAK inhibitor in the treatment of anti-MDA5 antibody-positive dermatomyositis patients. Identifier NCT04966884.
- Baricitinib for cutaneous dermatomyositis. Identifier NCT05361109.
- Baricitinib in patients with relapsing or naïve dermatomyositis (BIRD). Identifier NCT04972760.
- A study to investigate the efficacy and safety of brepocitinib in adults with dermatomyositis (VALOR). Identifier NCT05437263.
- JAK inhibitors in lichen planus: a review of pathogenesis and treatments [published online ahead of print, 2022 Aug 29] J Dermatolog Treat 2022:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710. e2
- [CrossRef] [PubMed] [Google Scholar]
- Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-16. e4
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol Ther. 2018;31:e12656.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sarcoidosis of the skin: a review for the pulmonologist. Chest. 2009;136:583-96.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers in the diagnosis and prognosis of sarcoidosis: current use and future prospects. Front Immunol. 2020;11:1443.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of cutaneous sarcoidosis with tofacitinib: A case report and review of evidence for Janus kinase inhibition in sarcoidosis. JAAD Case Rep. 2021;16:62-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis. N Engl J Med. 2018;379:2540-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tofacitinib for cutaneous and pulmonary sarcoidosis: A case series. J Am Acad Dermatol. 2021;84:581-3.
- [CrossRef] [PubMed] [Google Scholar]
- Dramatic response of refractory sarcoidosis under ruxolitinib in a patient with associated JAK2-mutated polycythemia. Eur Respir J. 2018;52:1801482.
- [CrossRef] [PubMed] [Google Scholar]
- Resolution of cutaneous sarcoidosis after Janus kinase inhibitor therapy for concomitant polycythemia vera. JAAD Case Rep.. 2019;5:360-1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Steroid-resistant sarcoidosis treated with baricitinib. Ann Rheum Dis. 2020;79:1259-60.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of type 1 immunity with tofacitinib is associated with marked improvement in longstanding sarcoidosis. Nat Commun. 2022;13:3140.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Graft-versus-host disease: Part I. Pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-534.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Insights into the role of the JAK/STAT signaling pathway in graft-versus-host disease. Ther Adv Hematol. 2020;11:2040620720914489.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800-10.
- [CrossRef] [PubMed] [Google Scholar]
- FDA approval summary: ruxolitinib for treatment of chronic graft-versus-host disease after failure of one or two lines of systemic therapy. Oncologist. 2022;27:493-500.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of baricitinib in refractory chronic graft-versus-host disease (cGVHD): Preliminary analysis results of a phase 1/2 study. Blood. 2020;136(Supplement 1):1.
- [Google Scholar]
- Phase I/II Study of Pacritinib, A JAK2/IRAK1/CSF1R inhibitor, in refractory chronic graft-versus-host disease (cGVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Identifier NCT05531786.
- A Phase I/II gvhd prevention trial combining pacritinib with sirolimus-based immune suppression. Identifier NCT02891603.






