Translate this page into:
Lack of antinuclear antibody in children with atopic dermatitis
2 Departments of Internal Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Sandipan Dhar
H No 1-N-14, Vigyan Nagar, Kota-324005
India
| How to cite this article: Dhar S, Kanwar AJ, Deodhar SD. Lack of antinuclear antibody in children with atopic dermatitis. Indian J Dermatol Venereol Leprol 1997;63:5-8 |
Abstract
Antinuclear antibody (ANA) was assayed in 76 children with atopic dermatitis (AD) of which 46 were males and 30 females. Their ages ranged from 6 months to 12 years (mean 3.4 years). Age at onset of AD ranged from 2 months to 5.5 years (mean 1.9 years) and its duration ranged from 4 months to 4 years (mean 1.2 years). While facial lesions were present in 56 (73.3%) patients, 49 (64.5%) patients had predominant involvement of extensors. As per severity score designed by Rajka and Langerland, 31 (40.8%), 42 (55.3%) and 3 (3.9%) patients had mild, moderate and severe diseases respectively. History of photosensitivity was present in 6 (7.9%) patients. Serum samples were positive for ANA in a very low titre (1:20) in 2/6 patients with facial lesions. However LE cell, rheumatoid factor and C-reactive proteins were negative and serum complement levels were within normal limits.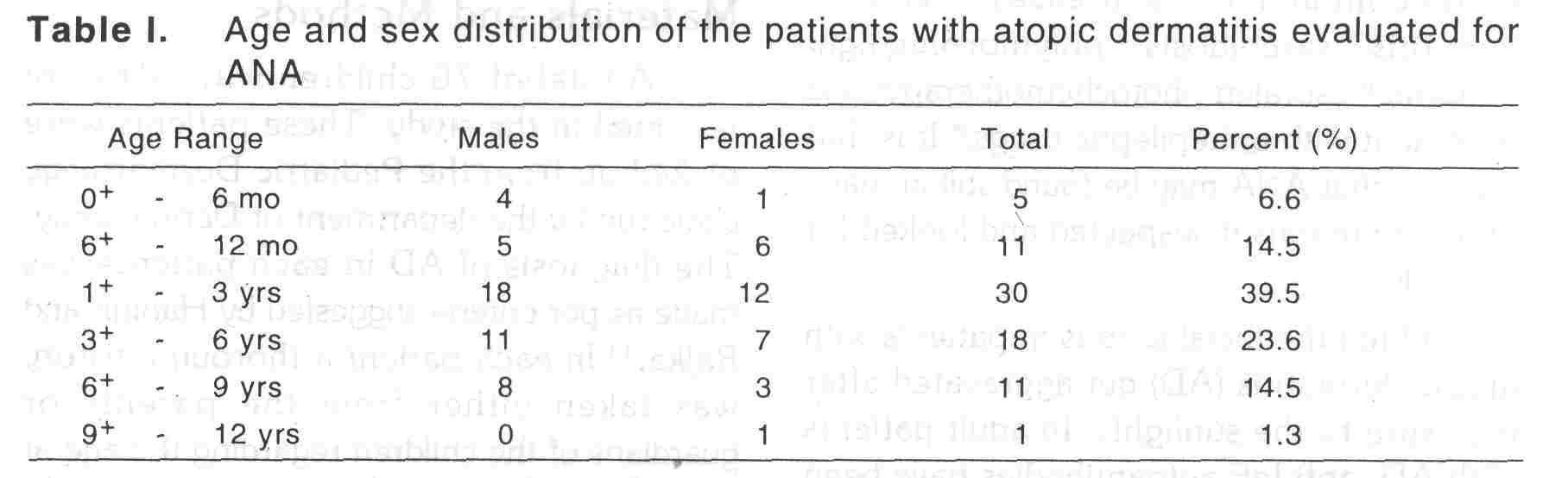

Introduction
Antinuclear antibody (ANA) is the hallmark for the diagnosis of SLE[1] and is found in a host of other autoimmune disorders.[1],]2] Other conditions where ANA has been found to be positive are viral infections, chronic inflammatory diseases,[3],[4] chronic hepatitis,[3] sarcoidosis,[5] polymorphic light eruption,[6] psoralen photochemotherapy[7] and treatment with antiepileptic drugs.[8] It is thus possible that ANA may be found still in many other conditions if suspected and looked for carefully.
Often the facial lesions in patients with atopic dermatitis (AD) get aggravated after exposure to the sunlight.[9] In adult patients with AD, anti IgE autoantibodies have been demonstrated[10] suggesting an ongoing autoreactive phenomenon in these patients. This is further substantiated by finding ANA positivity in patients with AD in two Japanese series.[11],[12]
The aforesaid observation prompted us to look for ANA in patients suffering from AD and to find if there is any correlation between ANA positivity with the severity of AD and presence of facial lesions.
Materials and Methods
A total of 76 children with AD were recruited in the study. These patients were picked up from the Pediatric Dermatology clinic run by the department of Dermatology. The diagnosis of AD in each patients was made as per criteria suggested by Hanifin and Rajka.[13] In each patient a thorough history was taken either from the parents or guardians of the children regarding the age at onset, duration, history of any drug intake prior to evaluation, associated fever, joint pain, redness of eyes, photosensitivity etc. A meticulous examination was carried out in each patient and the extent of eczema, presence or absence of facial lesions, predominant sites of involvement were noted down. Severity of AD was measured in each patient usuing scoring system proposed by Rajka and Langeland.[14]
Antinuclear antibodies were examined using rat liver as substract fixed in acetone; using serum diluted in phosphate buffered saline and fluorescein isothiocyanate (FITC)-conjugated goat antihumag IgG. The presence of immunofluorescence at a serum dilution of greater than or equal to 1:40 was considered as significantly positive.
Serum samples were taken for estimation of ANA in 58 age and sex matched controls. The controls were selected from healthy normal children and children suffering from nonatopic disorders. Children suffering from disorders or on drug known to cause ANA positivity were excluded from the controls.
Blood samples were also tested for LE cell, rheumatoid factor, complement levels in patients and controls.
Results
The age ans sex distribution of the patients has been shown in [Table - 1]. Most of the patients were in the age group 1-3 years. Age at onset of AD and its duration ranged from 3 months to 5.5 years (mean 1.9 years) and 4 months to 4 years (mean 1.2 years) respectively. There was no history of fever, arthralgia or redness of the eyes in any of the patients evaluated. Neither there was any history of intake of durgs other that that for eczema in the precedding one month of the evaluation. While facial lesions were present in 56 (73.7%) patients, 49 (64.5%) patients had predominant involvement of extensors, As per severity score, 31 (40.8%), 42 (55.3%), 3 (3.9%) patients had mild, moderate and severe diseases. History of photosensitivity was present in 6 (7.9%) patients only, all having facial lesions and with predominant involvement of extensors.
Serum samples were positive (1:40) for ANA in only 2 (2.6%) of 6 patients, one showing speckled pattern and the other homogeneous pattern of fluorescence: Both the patients were females, had history of photosenstitivity, facial lesions, predominant extensor involvement and moderate dermatitis (severity score ′6′). Serum samples for ANA was negative in all other patients with AD. Other tests viz, LE cell, rheumatoid factor, C-reactive protein were, however, negative in them. Serum comlement levels were also within normal limits in both ANA positive and ANA negative patients.
Blood sample was positive for ANA in only one healthy male child among the controls. However, other tests eg. LE cells, Creative protein, rheumatoid factor etc were negative and serum complement levels were normal in him.
Discussion
In a study from Japan, Taniguchi et al[11] found ANA to be positive in adult (age 15-30 years) patients with AD in 34% and 26% respectively usuing two different methods and it was significantly higher than control values i.e., 25% and 16.7%. Facial lesions resembling photosensitive butterfly erythema of SLE was present in 81.2% of their patients with AD having positive ANA. In our study, however, both the patients with positive ANA had mild facial dermatitis.
In another study from Japan by Tada et al[12] positive ANA was found in 25.8% of patients with AD (titres 1:40 to 1:640) against 12.1% of controls. However, these were not statistically significant.
In both studies frequency of positivity and its titre were higher in females. In the present study, both the patients with positive ANA were females. Neither of the two patients with low positive ANA had any abnormality in other collagen profiles eg, LE cells, rheumatoid factor, C-RP, serum complement levels etc. This is in corroboration with observations by Japanese workers.[11],[12]
Although the age of patients in the study by Tada et al ranged between 5 to 49 years, mean age was 19.0 ± 7.4 years. So, most of the patients were presumably adults.
The substrate used for ANA testing was Hep-2 cells in the Japanese studies. We, however, used acetone fixed rat liver as substrate. Therefore the results of the present study are difficult to compare with those carried out by Japanese workers.
However, we conclude that positivity of ANA is not feature of childhood AD in north India. This could be either because of a less severe AD seen in India[15],[16] or it may be related to many unusual features of AD observed in India.[15] However, its exact significance is too early to evaluate. Whether it is marker of severity of AD or it is just a special feature seen in Japanese patients needs further confirmation by more studies from other parts of the world.
| 1. |
Tan EM. Antinuclear antibodies: Diagnostic marker for autoimmune disease and probes for cell biology. Adv Immunol 1989;44:93-151.
[Google Scholar]
|
| 2. |
Davis JS, Antinuclear antibodies (ANA). In: Kelly WN, Harris ED, Ruddy S, Stedge CB, eds. Textbook of rheumatology. Philadelphia: WB Saunders, 1981:691-709.
[Google Scholar]
|
| 3. |
Wilson MR. Antinuclear antibodies and cytoplasmic antibodies in lupus erythematosus. In: Wallace DJ, Dubois EL, eds. Dubois Lupus erhthematosus. Philadelphia: Lea & Febiger, 1987:227-43.
[Google Scholar]
|
| 4. |
Sontheimer RD, Deng JS, Gilliam JN. Antinuclear and anticytoplasmic antibodies. J Am Acad Dermatol 1983;9:335-43.
[Google Scholar]
|
| 5. |
Veien NK, Hardt F, Bendixen G, et al. Immunological studies in sarcoidosis: a comparison of disease activity and various immunological parameters. Ann NY Acad Sci 1976;278:47-51.
[Google Scholar]
|
| 6. |
Murphy GM, Hawk JLM. The prevalence of antinuclear antibodies in patients with apparent polymorphic light eruption. Br J Dermatol 1991;125:448-51.
[Google Scholar]
|
| 7. |
Picascia DD, Rothe M, Goldberg NS, et al. Antinuclear antibodies during psoralens plus ultraviolet A (PUVA) therapy-are the worthwhile? J Am Acad Dermatol 1987;16:574-7.
[Google Scholar]
|
| 8. |
De Giorge CM, Rabinowicz AL, Olivas RD. Carbamazepine induced antinuclear antibodies and systemic lupus erythematosus like syndrome. Epilepsia 1991;32:128-9.
[Google Scholar]
|
| 9. |
Frain-Bell W, Scatchard M. The association of photpsensitivity and atopy in the child. Br J Dermatol 1971;185:105-10.
[Google Scholar]
|
| 10. |
Twena DM, Marshal JS, Maney MR, et al. A survey of nonatopic and atopic children and adults for the presence of anti-lgE auto antibodies. Clin Immunopathol 1989;53:40-51.
[Google Scholar]
|
| 11. |
Taniguchi Y, Yamakani A, Sakamoto T, et al. Positive antinucle antibody in atopic dermatitis. Acta Derm Venereol (Stockh) 1992; suppl 176:62-4.
[Google Scholar]
|
| 12. |
Tada J, Toi Y, Yoshioka T, et al. Antinuclear antibodies in patients with atopic dermatitis and severe facial lesions. Dermatology 1994;189:38-40.
[Google Scholar]
|
| 13. |
Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980; suppl 92:44-7.
[Google Scholar]
|
| 14. |
Rajka G, Langeland T. Grading of severity of atopic dermatitis. Acta Derm Venereol (Stockh) 1989;suppl 144:13-4.
[Google Scholar]
|
| 15. |
Kanwar AJ, Dhar S. Severity of atopic dermatitis in India. Br J Dermatol 1994;131:733-4.
[Google Scholar]
|
| 16. |
Dhar S, Kanwar AJ. Grading of severity of atopic dermatitis in north Indian children. Ind J Dermatol 1995;40:67-72.
[Google Scholar]
|





