Translate this page into:
Laser-assisted surgery and bioscaffold for the treatment of rhinophyma
2 Special Needs and Maxillo-Facial Surgery Unit, Hospital “Guglielmo da Saliceto”, Piacenza, Italy
Correspondence Address:
Elisabetta Merigo
Special Needs and Maxillo.Facial Surgery Unit, Hospital “Guglielmo da Saliceto”, Via Taverna 49, 29100 Piacenza, Italy
| How to cite this article: Merigo E, Cella L, Oppici A, Fornaini C. Laser-assisted surgery and bioscaffold for the treatment of rhinophyma. Indian J Dermatol Venereol Leprol 2018;84:629-631 |
Sir,
Rhinophyma (from the Greek language, “rhis” meaning “nose” and “phyma” meaning “growth”) is a rare, progressive, and disfiguring proliferative disorder of the nose characterized by a bulbous, cosmetic nasal deformity capable of producing a distortion in the aesthetic subunits of the lower nose and alar thickening sometimes leading to obstruction of the external nasal valves.[1]
The exact etiology of rhinophyma is still unknown even if it is believed to be multifactorial in origin, with the principal pathogenesis related to an unregulated superficial vasodilation.[1]
Rhinophyma is the result of hyperplasia and fibrosis of the sebaceous glands,[2] connective tissue, and blood vessels.[3] In literature, two forms of rhinophyma are described – a common form, peculiar to rosacea, and a “fibrous variant.”[3],[4]
Topical antibiotics or retinoids are ineffective for rhinophyma and only surgery can correct this condition. Cases of patients treated with scalpel excision, electrosurgery, or laser have been reported in the literature, with the last mainly used for ablative resurfacing.
Diode lasers emit a very wide range of wavelengths, in particular, the visible red and near-infrared. The wavelength used for the patient reported here was 808 nm (near infrared), which is frequently described for its surgical use.[5]
The bioscaffold used in the patient (Matriderm® - Skin and Health Care AG, Billerbeck, Germany) was a single-use, biocompatible, three-dimensional matrix composed of native, structurally intact collagen fibrils and elastin for supporting dermal regeneration. Its utilization as a scaffold in skin reconstitution is based on its hemostatic properties as well as its ability to promote cell growth and vascularization.[6] This bioscaffold constitutes a matrix used by tissues to repair and regenerate themselves, thus promoting, regulating and speeding up these processes; moreover, it reduces the scar effect by amplifying the regenerative process.
Here, we present the case of a 75-year-old man with positive anamnesis for myasthenia who presented with a large bulbous growth on his nose which was reported by the patient as rapidly growing since 3 months. Physical examination showed an exophytic bulbous mass on the nose with obliteration of the normal rhinal contour with nodular-cystic areas and large dilated pores [Figure - 1]. Because of his embarrassment with cosmetic appearance, the patient decided to undergo a surgical intervention never done before.
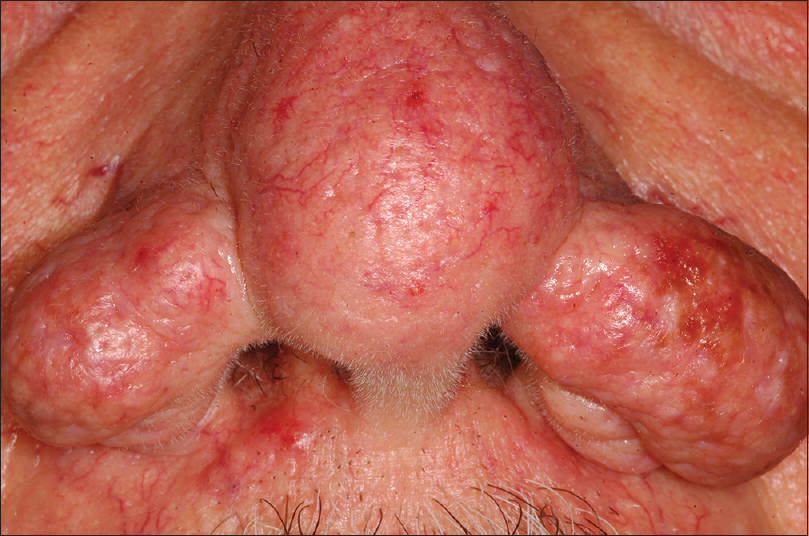 |
| Figure 1: Clinical appearance of the rhinophyma patient before surgery |
Surgery was performed at the Dentistry, Special Needs and Maxillo-Facial Surgery Unit of the Hospital “Guglielmo da Saliceto” of Piacenza (Italy). Surgery was planned and drawn directly on the patient's skin with a black pen to help the laser cut.
With previous asepsis and local anesthesia by 1% articaine and epinephrine regional perinasal nerve block, an 808-nm diode laser (Lasersmile – Fotona – Slovenia) was used with the following parameters: 320 μm optic fibre in continuous wave (CW) used in contact mode with a power of 2, 5 W (Power density: 3125 W/cm2). The hypertrophic tissue was cut at three different times [Figure - 2]a and b].
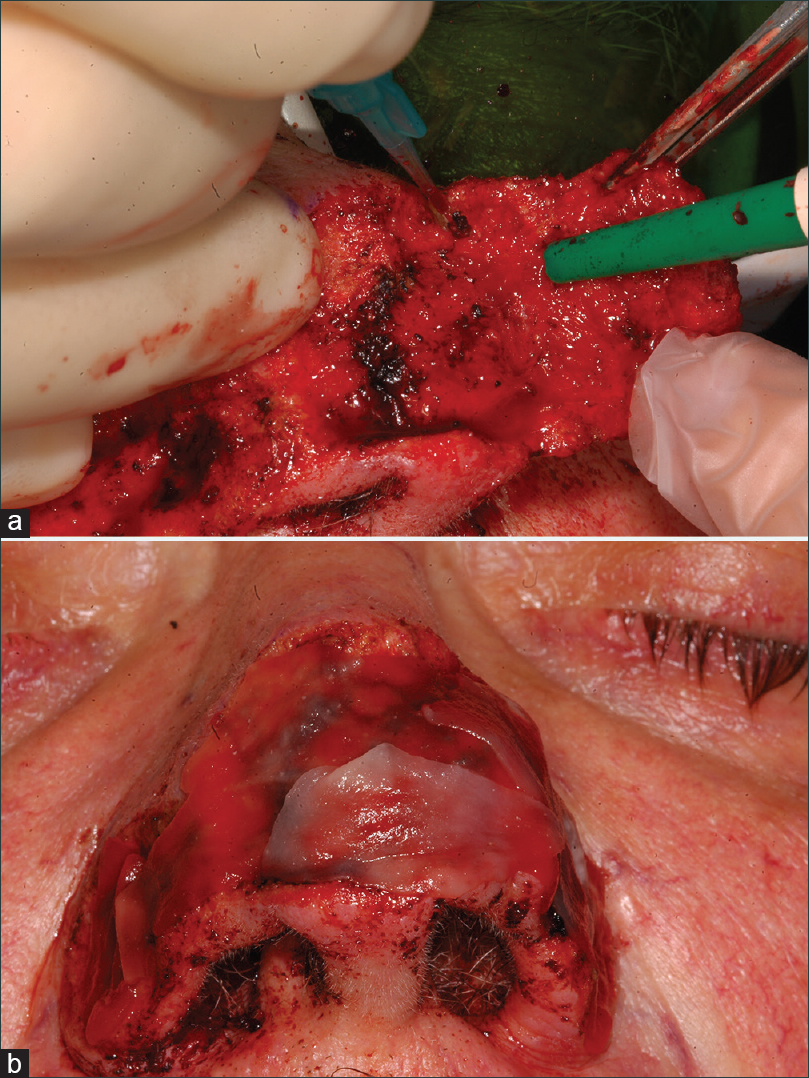 |
| Figure 2: (a and b) Surgical laser-assisted treatment with 808 nm diode laser along with application of Matriderm® |
The 808-nm diode laser was used because of its interactions with chromophores such as hemoglobin and melanin able to well absorb the infra-red laser emission. Owing to the intrinsic affinity of 808 nm wavelength for haemoglobin, laser-assisted surgery was performed in a clean surgical field due to good hemostasis (fine bipolar diathermy was only used to cauterize occasional pulsatile bleeding big vessels); moreover, the biostimulating effects led to fast and good healing.
After the removal of the hypertrophic tissue, the surgical site appeared bloodless and ready for the application of the bovine bioscaffold without skin graft.
The bioscaffold was removed from its package under sterile conditions and was cut (in its still dried form) on the basis of the nose surface and rehydrated in physiological saline solution. Subsequently, it was positioned on the nose surface without fixing by stitches but only using its own capacity to bind the blood clot to fixate itself.
The bioscaffold used here had a dehydrated dermis and dimensions of 52 × 74 mm with a thickness of 1 mm; it exhibited traction strength due to its dry and inelastic structure but after its immersion in physiological solution it became elastic and plastic without changing shape and dimension. After this step, there are only few minutes (about 5–10) to change the position of the dermal patch. In fact, after this time, the attempt to modify the position of the bioscaffold may cause laceration in the traction point. After its positioning, it is necessary to wait for 10 min to achieve spontaneous adhesion to the site, favoring haemostasis. After 1–2 hours of homing, it is very difficult to identify the bioscaffold due to its metabolization by the surgical bed.
After surgery, the patient came to our unit every day for 2 weeks to check the healing process. He never reported pain, neither immediately after surgery nor during the subsequent days.
Re-epithelialization occurred gradually without any discomfort and it was fully completed with the absence of dyschromic skin 6 months after surgery [Figure - 3]a and b].
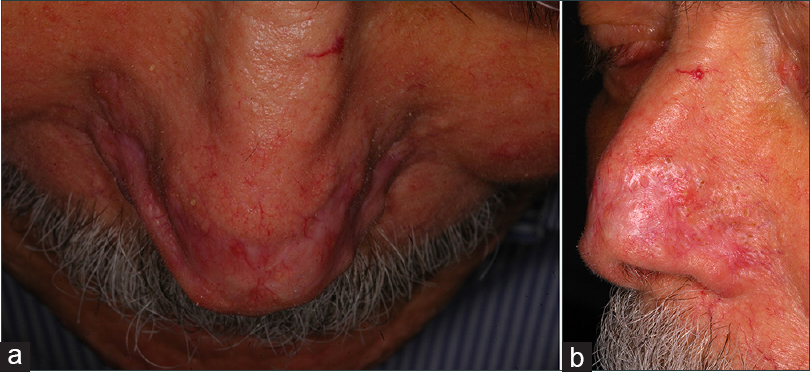 |
| Figure 3: (a and b) Complete healing without recurrence 6 months after surgery |
The use of diode laser excisional therapy for the treatment of rhinophyma has several advantages, with hemostasis being the main advantage. In fact, surgical field was not bleeding except for the bigger vessels damaged during tissue removal, which required hemostasis with bipolar cautery. This represents an advantage for the operator, allowing a better view of the surgical site with respect to all the structures and performing a more precise cut design.
As reported in literature, laser surgery in the tissues surrounding the surgical site, due to the scattering phenomena, may create some photochemical effects allowing a biomodulating reaction possibly linked to the stimulation of wound healing, reduction of inflammation, and analgesic effect; these may be the reasons why the patient did not report any pain during, immediately after surgery, and postsurgically, as well as can be the cause of the reduced healing time.
Laser cut gives also a bactericidal effect possibly responsible for the low risk of infection; this antimicrobial aspect may be enhanced by the properties of Matriderm®.
Wavelengths other than 808 nm can be used for this surgical application: Nd: YAG laser (wavelength 1064 nm) and KTP laser (wavelength 532 nm), owing to their similar affinity for melanin and hemoglobin, should give the same good bleeding control, providing biomodulation effects and favouring the healing process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Laun J, Gopman J, Elston JB, Harrington MA. Rhinophyma. Eplasty 2015;15:ic25.
[Google Scholar]
|
| 2. |
Macdonald JB, Nguyen XH. Images in clinical medicine: Rhinophyma. N Engl J Med 2012;367:1838.
[Google Scholar]
|
| 3. |
Lazzeri D, Larcher L, Huemer GM, Riml S, Grassetti L, Pantaloni M, et al. Surgical correction of rhinophyma: Comparison of two methods in a 15-year-long experience. J Craniomaxillofac Surg 2013;41:429-36.
[Google Scholar]
|
| 4. |
Lazzeri D, Agostini T, Spinelli G. Optimizing cosmesis with conservative surgical excision in a giant rhinophyma. Aesthetic Plast Surg 2013;37:125-7.
[Google Scholar]
|
| 5. |
Fornaini C, Merigo E, Vescovi P, Lagori G, Rocca J. Use of laser in orthodontics: Applications and perspectives. Laser Ther 2013;22:115-24.
[Google Scholar]
|
| 6. |
De Angelis B, Gentile P, Agovino A, Migner A, Orlandi F, Delogu P, et al. Chronic ulcers: MATRIDERM® system in smoker, cardiopathic, and diabetic patients. J Tissue Eng 2013;4:2041731413502663.
[Google Scholar]
|
Fulltext Views
3,458
PDF downloads
2,100





