Translate this page into:
Lasers for vascular lesions: Standard guidelines of care
Correspondence Address:
M Kumaresan
Department of Dermatology, PSG Hospitals, Coimbatore, Tamil Nadu
India
| How to cite this article: Srinivas C R, Kumaresan M. Lasers for vascular lesions: Standard guidelines of care. Indian J Dermatol Venereol Leprol 2011;77:349-368 |
Abstract
Introduction: Lasers are a good therapeutic tool for congenital and acquired vascular lesions. Technological advances in lasers have reduced the adverse effects and increased the efficacy. Machines: Among the various lasers used for treating vascular lesions, pulsed dye laser (PDL) has the best efficacy and safety data. The other machines that are widely available are Nd:YAG laser and intense pulse light (IPL). Rationale and scope of guideline: Much variation exists in different machines and techniques, and therefore, establishing standard guidelines has limitations. The guidelines recommended here indicate minimum standards of care for lasers on vascular lesions based on current evidence. Physician Qualification: Laser may be administered by a dermatologist, who has received adequate background training in lasers during post-graduation or later at a center that provides education and training in lasers, or in focused workshops, which provide such trainings. He/she should have adequate knowledge of the lesions being treated, machines, parameters, cooling systems, and aftercare. Facility: The procedure may be performed in the physician's minor procedure room with adequate laser safety measures. Indications: PWS, hemangioma, facial telangiectasia, rosacea, spider angioma, pyogenic granuloma, venous lakes, leg veins. Contraindications: Absolute: Active local infection, photo-aggravated skin diseases, and medical conditions. Relative: Unstable vitiligo, psoriasis, keloid and keloidal tendencies, patient on isotretinoin, patient who is not cooperative or has unrealistic expectation. Patient Selection: Patient selection should be done after detailed counseling with respect to the course of lesions, different treatment options, possible results, cost, need for multiple treatments, and possible postoperative complications. Treatment Sessions: The number of treatments per lesion varies from 2 to 12 or more at 6-8 week intervals. All lesions may not clear completely even after multiple sessions in many cases. Hence, a realistic expectation and proper counseling is very important. Laser parameters: Laser parameters vary with area, type of lesion, skin color, depth of the lesion, and machine used. A test spot may be performed to determine individual specifications. Complications: Pain, edema, purpura, bleeding, scarring, postinflammatory hyperpigmentation/hypopigmentation, and atrophy changes.Introduction
Lasers were first used to treat cutaneous pathologies in 1963 by Dr. Leon Goldman. Although Goldman originally used a ruby laser for skin treatment, argon and carbon dioxide continuous wave (CW) lasers soon became the main treatment modalities for the first generation of dermatologic lasers. [1] Early development in laser was focused on treating congenital lesions. Later, lasers were found to be effective in treating acquired vascular lesions also. Significant advances such as dynamic surface cooling and extended pulse duration have resulted in enhanced clinical results and minimized adverse effects.
Rationale and Scope of the Guidelines
Although laser and light sources are effective and safe modalities, proper guidelines and safe practices should be adopted by practitioners to achieve satisfactory results and avoid side effects. These guidelines focus on patient selection and treatment protocol in order to provide safe and effective treatment. The guidelines will discuss the different types of laser or IPL used, indications, contraindications, case selection, factors determining the outcome such as the vessel′s characteristics, site, color, depth, the Fitzpatrick skin type, age of the patient, size of the lesion, laser spot size, etc. Much variation exists in different machines and techniques, and therefore, establishing standard guidelines has limitations. The guidelines recommended here indicate minimum standards of care for lasers on vascular lesions based on current evidence. It is emphasized hereby that this technology is new and rapidly evolving, and therefore, these guidelines are based on the available evidence so far and may need to be reviewed periodically as the technology evolves. The most important aspect is that available vascular lasers are more suitable for western skin types and the parameters used in western skin may not always suit the Indian skin types. However, published data with respect to Indian skin are limited, and therefore, the guidelines are to be treated as exploratory advisories at best especially for PWS. It is also further recommended that as more experience is gained with the use of these lasers, the recommended parameters may need to be revised.
Principles of Lasers for Vascular Lesions
Laser physiology: Lasers and non-coherent intense pulse light sources (IPL) are based on the principle of selective photothermolysis. The target chromophore in vascular lesions is the oxyhemoglobin present in the red blood corpuscles (RBCs) which circulates in the blood vessels. Oxyhemoglobin has three major absorption peaks at 418, 542, and 577 nm. [1] Optimal absorption is within 577?600 nm range [Figure - 1]. [1] After absorption of laser by oxyhemoglobin, light energy is converted to thermal energy. Thermal energy diffuses radially within the blood vessel leading to selective microvascular damage, through photocoagulation and mechanical injury. The end result is thrombosis of the blood vessels. If the pulse duration is more than the thermal relaxation time (TRT), non-selective thermal damage of perivascular connective tissues occurs leading to tissue destruction and scarring.
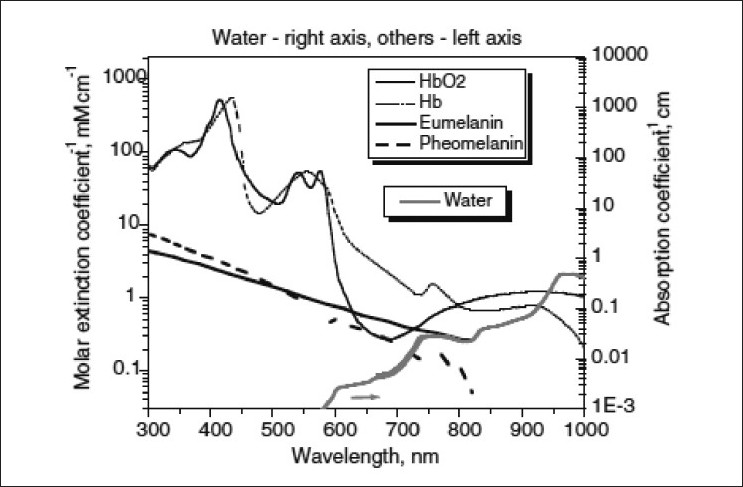 |
| Figure 1: Absorption spectrum of various chromophores |
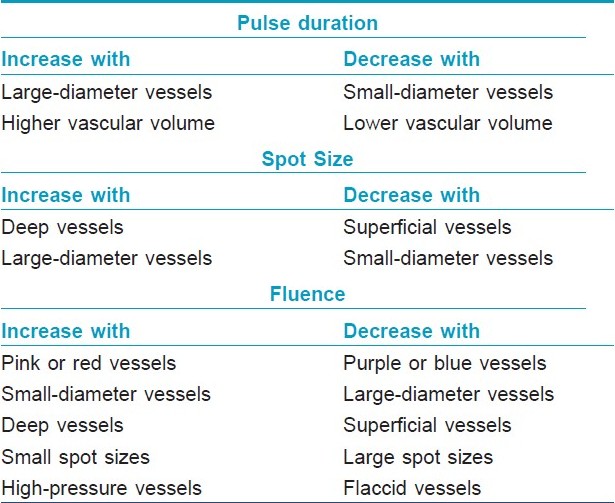
Several factors such as size of vessels, depth of the lesion, area of body treated, laser spot size, skin type, etc. affect the absorption of laser as follows.
Diameter of vessels: Vessels with a diameter of 10?100 μm have TRT of 1?10 ms. [2] Blood vessels with diameter >100 μm have a higher TRT, and therefore, require longer pulse duration. [2]
Depth of vessel : The location of the blood vessels should also be taken into account. The more superficially located lesions (papillary dermis) will respond to standard wavelengths (577, 585 nm) but the deeper vessels will need lasers with longer wavelength (up to 600 nm). [3] Longer wavelength allows deeper penetration and longer pulse width allows treatment of large caliber vessels. [3] When considering vessel depth, it is important to consider the deepest part of the vessel and not its closest distance from the epidermis. [4]
Site of lesions : Vessels on the leg are located deeper and contain more deoxyhemoglobin, hence they absorb the laser light in the 800?1200 nm wavelength spectrum. [5] Infrared wavelengths tend to be more effective in treating deeper blue vessels while shorter wavelengths are more effective for superficial red telangiectasias. [5] Lesions on face and upper trunk respond better than those in lower trunk and legs. [2] Areas prone to scarring such as, anterior chest, neck or areas where the skin is delicate such as periorbital region and neck requires a reduction in fluence of 10?20%. [2] Vascular malformation located on the first and third branches of trigeminal nerve respond to the laser treatment better than those over the second branch. [5]
Age of patients: Young people respond better than elderly since the blood vessels are smaller and more superficially located. [2]
Skin type: Darker skin types require longer pulses, longer pulse intervals and higher fluence, since epidermal melanin absorbs the laser energy. [3]
Spot size : Laser beam with larger spot size will have a deeper penetration and reduces the total treatment time. [3] Spot sizes that are small result in greater scatter of laser energy, and are therefore, not as effective in thermo coagulating large or deep vessels. Spot sizes that are large scatter little light and may deliver greater energy to the desired target, resulting in more photocoagulation and swelling. [3] One should not underestimate the dramatic increase the energy delivered to tissues upon increasing spot size. When in doubt, smaller spot sizes are recommended, with manipulation of the fluence to achieve the desired energy at the target level. [4]
Fluence: Fluence is the energy of laser light delivered per unit area. Selection of fluence is based mainly on vessel color, but other determinants, including vessel size, vessel depth, spot size setting, and vessel pressure, are also of importance. [4] Purple and blue vessels tend to absorb light energy more than pink and red vessels, and therefore, require less fluence. [4] Special care must be taken to ensure that excessive fluence is not employed for large spot sizes to prevent excessive tissue damage. Smaller vessels have less light absorbency because of the small amount of chromophore, and small spot sizes are associated with greater light scatter, necessitating a compensatory higher fluence (i.e., greater light intensity). [4] Vessels under greater intravascular pressure, such as on the nose or legs, require higher fluences to achieve effective thermocoagulation than those with less intravascular pressure. [4]
In view of these variabilities in laser parameters and possibility of variations in individual responses, it has been suggested that a test spot on a small, representative area may be performed initially, before treating the entire lesion. [5]
Role of Epidermal cooling : High energy is required to thermocoagulate the vessels located deep in the skin, and hence epidermis should be protected by cooling to minimize the damage to keratinocytes and melanocytes. Cooling is, therefore, an integral part of vascular lasers. Cooling can be done by different methods such as cryogen spray, cold sapphire contact hand pieces or by blowing pre-cooled air across the skin surface. The dynamic cooling device (DCD) is either an add on device or integrated within the laser. The laser software allows the user to set the DCD spray and delay settings and the cryogen sprays the skin before the laser pulse to cool the skin. While cooling helps minimize epidermal damage, it may, paradoxically, reduce the efficacy of lasers, by its blanching effect on the underlying blood vessels.
Classification of Vascular Lasers
There are several types of lasers used in skin laser surgery. Older laser technologies such as the continuous wave (CW) lasers of CO 2 and argon have been largely replaced with quasi-CW mode lasers and pulsed laser systems.
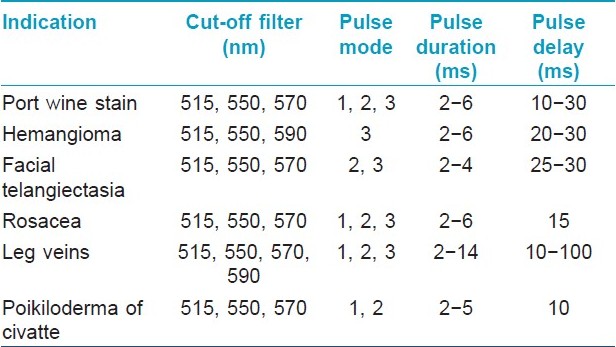
The wavelength peaks of the laser light, pulse durations and how the target skin tissue absorbs this, determine the clinical applications of the laser types [Table - 1].

Several vascular lasers (such as argon, tunable dye, copper vapor, krypton lasers), which were used in the past are no longer used as they pose a higher risk of complications such as dyschromia (hypopigmentation or hyperpigmentation) and scarring.
Flash-Lamp Pumped Pulse Dye Laser
PDL is the most commonly used laser for vascular lesions. PDL emits a pulsed beam of yellow light at 585 nm, powered by a flash lamp. [6] The lasing medium is a fluorescent organic dye dissolved in a liquid solvent and housed in a transparent cell. [7] The dye′s chemical structure, solvent, and additives used dictate the operating lifetime, which is limited, due to decomposition of dye, solvent, and additives when exposed to heat and intense light. [7] Rhodamine 6G dye is efficient and has a long life time. The cell containing the dye is surrounded by a flash lamp capable of producing pulse duration of 450 μs. [7] The pulse duration of 450 μs is at the lower end of the range of TRT of skin vasculature, [8] which is 200?3000 μs for vessels with diameter of 10?40 μm. [7] At 585 nm and 450 μs PDL penetrates to a depth of 0.2 mm below the dermo-epidermal junction, which can be increased to 0.5?1.2 mm by increasing the wavelength. [7] Use of the first-generation PDLs with short pulse durations, small spot size, high fluence, and short wavelengths produced very high complication rates. A common complication is post treatment purpura which appears immediately and can last for 7?14 days, which may be cosmetically unacceptable. [9] The other common side effects are hyper pigmentation, hypopigmentation, hypertrophic scarring, dermal and epidermal atrophy.
The more recent PDLs have large spot sizes (elliptical 2 Χ 7 mm) and longer wavelengths (590, 595, and 600 nm) for greater depth of penetration. They provide longer pulse durations (1.5-40 ms) for better matching to the feeding vessel′s size, and epidermal cooling to reduce complications and allow safe use of higher fluences. Treatment is performed with fluences ranging from 3 to 10 J/cm 2 and spot sizes of 2, 3, 5, 7, or 10 mm and a 2 Χ 7 mm elliptical spot size. The high peak energy of short pulse duration PDL is thought to produce immediate purpura by a photoacoustic shattering of capillary walls with resultant leaking of red blood cells into extravascular tissue. [9] Longer pulse duration PDL achieves lower peak energy, and the heat is applied to blood vessels more slowly. This slower application of thermal energy in turn eliminates or minimizes any photoacoustic effects to capillary walls with less red blood cells leakage, and hence, no purpura, while maintaining clinical efficacy. [9]
Studies have found that increasing pulse durations, with a larger number of subpulses provide better selectivity between large (>100 μm) and small (<50 μm) vessels, resulting in a higher purpura threshold. [10] Tanghetti et al., [10] found that increasing the number of subpulses in an extended pulse format resulted in a monotonic increase in purpura threshold, allowing purpura-free treatment at higher fluences and this effect was more pronounced with longer pulse durations, such as 20 and 40 ms and less pronounced at shorter pulse durations, such as 2-10 ms. The new generation PDL uses a novel pulse structure where each macropulse is subdivided into 6-8 micropulses, so that with single pass treatments, greater total fluence can be delivered to tissues in a gentle fashion with evenly spaced micropulses that mitigate the risk of purpura. [11]
PDL can be safely used in children and infants. [12] Multiple repeat treatments may be preferred to minimize complications in children. [12] PDL was initially developed for port wine stains, but it is now used for several acquired vascular lesions such as telangiectasia, pyogenic granuloma, venous lake, cherry angioma, and poikiloderma of civatte. PDL is difficult to maintain and service. The dye and the lamp have to be checked at the end of 1 year and have to be replaced if necessary. The machine has to be switched on at least once a day in order to circulate the dye within the machine for proper functioning.
Neodymium :Yttrium-Aluminum-Garnet (ND:YAG) Laser
Nd:YAG laser has a wavelength of 1064 nm. This wavelength has a deeper penetration, a lower absorption by hemoglobin and the possible better absorption by deeper blue spider veins than the traditionally used shorter wavelengths (KTP, pulsed dye, IPL, diode). [5] The 1064-nm Nd:YAG laser is able to create a coagulation effect at a depth of 5?6 mm [13] and can, therefore, treat moderately deep, large caliber vessels, and feeding reticular veins. [14] The ratio of melanin to blood absorption is similar at 585 and 1064 nm, whereas the absolute values of absorption and scattering coefficients are considerably lower at 1064 nm as compared with 585 nm. [15] The absorption coefficient of blood at 1064 nm is 0.4/mm, which is much higher than that of the surrounding dermis (0.05/mm) at the same wavelength. This difference in absorption coefficients provides treatment selectivity of deep blood vessels. [16],[17] Lower absolute values of blood absorption at 1064 nm may be compensated by increasing the fluence. The increase in the treatment fluence does not necessarily cause damage to epidermis, because the absolute absorption of melanin is lower at 1064 nm as well. [15],[16],[17]
Nd:YAG system which is able to control depth of penetration through appropriate spot selection (3, 5, 7, and 10 mm), with variable pulse duration (0.1 - 300 ms) to adjust to different vessel diameters, high fluences (up to 300 J/cm 2 ) and optimal cooling systems to avoid pain or burning, should theoretically be able to treat any vascular lesion both superficial and deep or thick. However, some Nd:YAG devices have a limitation of parameter combinations and pulse durations, non-optimal cooling systems, and uneven fluence distribution ? this restricts their utility in the treatment of superficial or facial vascular lesions, as pain or risk of burning are common events.
Nd:YAG lasers are used to treat rosacea, facial telangiectasia, poikiloderma of Civatte, hemangiomas of infancy, tuberous hemangiomas, flat, and tuberous port wine stains (PWS) and leg veins. The 3 and 5 mm spot sizes are suitable for superficial lesions such as telangiectasias, rosacea, poikiloderma of Civatte, flat hemangiomas. The larger 5 or 7 mm spot sizes are needed for thicker or deeper ones such as tuberous hemangiomas or PWS, leg veins. [4] Pulse durations should be selected according to the estimated vessel size of each lesion; 0.3?5 ms for thin, 10?25 ms for medium and 25?55 ms for thick vessels. Variable fluences (from 14 to 195 J/cm 2 ) are used. All the parameters should be individually adjusted according to personal experience and immediate reaction (clearing or color change on vessels, threshold effect on hemangiomas or PWS) on each treatment [Table - 2].
Intense Pulsed Light
The intense pulsed light (IPL) produces a non-coherent light beam with a spectrum of wavelengths from 500 to 1200 nm. Cut-off filters at 515, 550, 570, 590 nm are used for vascular lesions. These devices generate a variety of fluences in single or multiple pulse modes in which the pulse duration and pulse delay can be varied. It is not a laser but works on similar principles. Computer software provides flexibility in using these devices, not normally available in laser. These devices have a bigger spot size; hence larger areas can be treated efficiently with less discomfort. [2] The different filter settings of the IPL enable a higher selection of a broad range of vessel colors of the vascular system. The longer wavelength emitted by this system can penetrate deeply into the tissues, theoretically improving the clinical efficacy. By splitting the energy into two or three pulses with different pulse delays, the skin can be cooled between pulses. This results in fewer and negligible side effects. [2]
Because of the rapid divergence of the IPL beam, the hand piece must be in contact (or almost in contact) with the skin for effective treatment. [18] Therefore, the physician cannot observe the vessel response until the hand piece is raised off the skin. Also, the larger spot sizes, although ideal for covering large areas, also pose the risk of "large" side effects. [18] Additionally, one can be compromised in maneuvering IPLs in tight concave areas (i.e., nasal crease). Depending on the IPL design, treatment over firmer surfaces (i.e., dorsum of the nose) can result in vessel compression and ineffective treatment of telangiectasias. [18]
A number of studies have reported the successful use of the IPL for the treatment of PWS, [19],[20] leg telangiectasias, [21],[22] essential telangiectasias, [23] venous malformations, [24],[25] and poikiloderma of Civatte. [26],[27] Fluence adjustment varies in different systems and should be based on the manufacturers advice. Treatment efficacy varies between the IPL systems and all are not equally effective. Ideal parameters for reproducible results have not been established [Table - 3].
Fractional Photothermolysis
The 1550 nm wavelength emitted from the fractional photothermolysis laser largely targets tissue water and not melanin. [28] Therefore, non-specific thermal injury to the epidermis, which may induce scarring and hypopigmentation, is unlikely. [28] This may especially be beneficial for the treatment of Fitzpatrick skin type IV-VI. [28] Additionally, the ′′fractional′′ laser avoids bulk heating of the skin dermis, which is seen in conventional pulsed mid-infrared lasers. [1] By adjusting the optical focal depth and/or the energy of the laser, high local radiant exposure can be achieved. [29] Different tissue compartments (e.g., blood vessels, dermal melanin, and sebaceous glands) at various depths of the skin can be arbitrarily selected as the targets for photothermolysis. With an appropriate microthermal zone (MTZ) density, an effective macroscopic treatment can be achieved.
There are two hypotheses as to the targeting of dermal vascular structures. Fractional photothermolysis emits a wavelength that largely targets water. As water is a large component of blood vessels, irradiance at 1550 nm may lead to fractional photothermal microvascular destruction with clinical benefits. [28] Second, the microthermal zones of injury in the dermis produced by this device may randomly result in frequent direct hits to the dermal vasculature. [1] Laubach and colleagues [30] reported the histopathologic evidence of damage to dermal vasculature in patients undergoing fractional photothermolysis to support the latter hypothesis. It is likely that both hypotheses work synergistically to produce reduction in telangiectasias. Fractional photothermolysis has been reported to be effective in poikiloderma of Civatte, [28] residual hemangiomas, [31] and telangiectatic matting. [32]
Carbon Dioxide Laser (CO2)
The CO 2 laser emits radiation with a wavelength of 10,600 nm, which is absorbed by the water in biological structures. The laser energy destroys the integrity of the cellular structure by quickly heating and vaporizing the intracellular liquid. Target structures such as pigmentation or vascularization are of no importance for the CO 2 laser, [33],[34] which is unlike other kinds of lasers such as the Q-switched ruby laser or the pulsed dye laser. Carbon dioxide (CO 2 ) laser can be utilized in two distinct modes: a continuous wave or an ultrapulsed system. Continuous wave system produces a continuous beam of light of uniform energy, which produces rapid tissue vaporization. The continuous beam of light does not vary in power with time. There is a certain risk of scars due to the unspecific coagulation in the continuous mode. By holding the laser hand piece at a distance from the skin greater than the focal length of lens, the laser beam is defocused and produces a large spot size of 1?2 mm or more. [35] Vaporization, occurs so quickly that there is minimal transfer of thermal energy to adjacent tissues. In ultrapulsed CO 2 laser systems, high irradiance pulses with short duration are emitted with the goal of focusing the thermal damage to minimize the damage to surrounding tissue. [36] CO 2 lasers have been used to treat nodules and hypertrophy in PWS and pyogenic granuloma.
Physician Qualification
Any qualified dermatologist (DVD/ DNB/ MD) may practice vascular lasers. He should possess post-graduate qualification in dermatology and should have had specific hands on training in lasers either during post graduation or later at a facility, which routinely performs laser procedures under a competent dermatologist/plastic surgeon who has experience and training in using lasers. Adequate knowledge of different vascular lesions, their pathologies are necessary. He/she should have adequate knowledge about the laser physics, laser - tissue interaction and laser safety. Since parameters may vary in different machines, specific training with respective machine at either manufacturer′s facility or at another centre using the machine is recommended.
Facility
Laser treatment for vascular lesions can be performed in the dermatologist′s minor procedure room.
Anesthesia
Most adults will be able to tolerate the discomfort. Children may require sedation or anesthesia. General anesthesia can be considered in children who have extensive lesions or are uncooperative with topical or local anesthesia, particularly those who are under 10 years of age. Young children undergoing multiple painful treatments with inadequate anesthesia under restraint may develop phobic responses and this may result in poor compliance. Furthermore, a struggling child may compromise the clinicians′ ability to perform the procedure optimally. The type of anesthesia used will depend on the physician and the facility. Topical anesthesia (EMLA) or local anesthesia (1% lignocaine) may be used to reduce the discomfort. However, EMLA is not recommended for children below 6 months of age since excessive absorption on highly vascular surfaces may cause methhemoglobinemia leading to cerebral hypoxemia.
Preoperative Counseling and Case Selection
Detailed counseling with respect to the course of lesions, different treatment options, possible results, cost, need for multiple treatments, and possible postoperative complications, should be discussed with the patient. Patient should be provided brochures to study and also adequate opportunity to seek information. In particular, the following aspects need to be discussed specifically:
- The natural course of the lesions, particularly in congenital lesions and possible medical treatments should be informed.
- Possibility of natural involution should also be discussed.
- Evaluation of eye for possible glaucoma in PWS on face in the distribution of V1 (first branch of trigeminal nerve) dermatome lesions is essential.
- While treating lesions on the spine, intra spinal pathology to be ruled out.
- MRI scan of the head and neck may need to be done in relevant cases
- In vascular lesions on limbs, arterial and venous Doppler to rule out arterial versus venous ectasia and consultation with vascular surgeon as necessary may be done.
For PWS, it should be emphasized that the results in our skin types are not uniform and inconsistent. This is because lasers are rarely made for our skin types.
Informed Consent
Detailed consent form needs to be completed by the patient (format provided). Consent form should include information on the machine used; results and postoperative course expected and possible postoperative complications. Preoperative photography should be carried out in all cases.
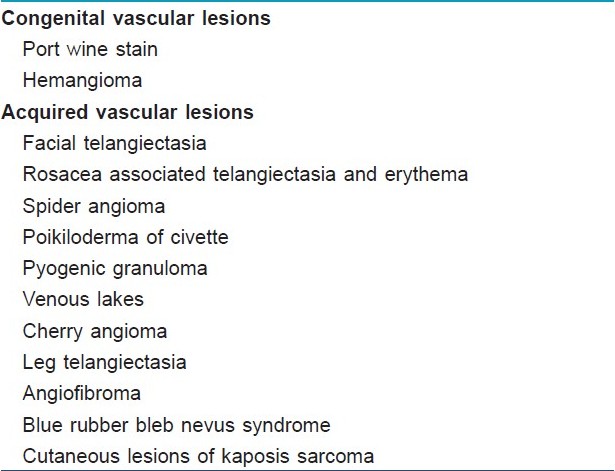
Indications for Vascular Lasers[Table - 4]
Contraindications for vascular lesions
Absolute:
- Active local infection,
- Photo-aggravated skin diseases and medical conditions.
Relative:
- Unstable vitiligo,
- Psoriasis.
- Keloid and keloidal tendencies,
- Patient on isotretinoin,
- Patient who is not cooperative or has unrealistic expectation.
References
- Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol 2003;49:1-31.
- Rothfleisch JE, Kosmann MK, Levine VJ, Ashinoff R. Laser treatment of congenital and acquired vascular lesions: update on lasers: A review. Dermatol Clin 2002;20:1-18.
- Garden JM, Bakus AD. Laser treatment of portwine stains and hemangiomas. Dermatol Clin 1997;15:373-38396.
- Groot D, Rao J, Johnston P, Nakatsui T. Algorithm for using a long-pulsed Nd:YAG laser in the treatment of deep cutaneous vascular lesions. Dermatol Surg 2003;29:35-42.
- Adamic M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPLS: Guidelines for care from the European Society for Laser Dermatology. J Cosmet Laser Ther 2007;9:113-24.
- Bucci J, Goldberg D. Past, present, and future: Vascular lasers/light devices. J Cosmet Laser Ther 2006;8:149-53.
- Herd RM, Dover JS, Arndt KA. Basic laser principles: Lasers in dermatology. Dermatol Clin 1997;15:355-72.
- Stratigos AJ, Dover JS. Overview of lasers and their properties. Dermatol Ther 2000;13:2-16.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg 2004;30:37-40.
- Tanghetti E. Pulsed dye laser extended pulse format effect on purpuric threshold. Lasers Surg Med 2003;15:74.
- Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulsed dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg 2007;33:1466-9.
- Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol 2008;58:261-85.
- Landthaler M, Hohenleutner U, Abd El Raheem TA. Therapy of vascular lesions in the head and neck area by means of argon, Nd:YAG, and flashlamp-pumped pulsed dye lasers. Adv Otorhinolaryngol 1995;49:81-6.
- Preeyanont P, Nimsakul N. The Nd:YAG laser treatment of hemangioma. J Clin Laser Med Surg 1994;12:225-9.
- Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol 1981; 77 :13-9.
- Landthaler M, Haina D,Brunner R, Waidelich W, Braun-Falco O. Neodynium-YAG laser therapy for vascular lesions. J Am Acad Dermatol 1986;14:107-17.
- Anderson RR. Optics of the skin. In: Lim HW, Soter NA, editors. Clinical photomedicine. New York: Marcel Dekker; 1993.P. 19-35.
- Ross EV, Smirnov M, Pankratov M, Altshuler G. Intense pulsed light and laser treatment of facial telangiectasias and dyspigmentation: Some theoretical and practical comparisons. Dermatol Surg 2005;31:1188-98.
- Raulin C, Schroeter CA, Weiss RA, Keiner M, Werner S. Treatment of port wine stains with a noncoherent pulsed light source: A retrospective study. Arch Dermatol 1999;135:679-83.
- Raulin C, Hellwig S, Schonermark MP. Treatment of a nonresponding port-wine stain with a new pulsed light source (PhotoDerm VL). Lasers Surg Med 1997;21:203-8.
- Green D. Photothermal removal of telangiectases of the lower extremities with the Photoderm VL. J Am Acad Dermatol 1998;38:61-8.
- Goldman MP, Eckhouse S. Photothermal sclerosis of leg veins. ESC Medical Systems, LTD Photoderm VL Cooperative Study Group. Dermatol Surg 1996;22:323-30.
- Raulin C, Weiss RA, Schonermark MP. Treatment of essential telangiectasias with an intense pulsed light source (PhotoDerm VL). Dermatol Surg 1997;23:941-5.
- Hellwig S, Schroeter CA, Raulin C. Treatment of essential telangiectasias with the Photoderm VL. Z Hautkr 1996; 71:44-7
- Raulin C, Werner S. Treatment of venous malformations with an intense pulsed light source (IPLS) technology: A retrospective study. Lasers Surg Med 1999;25:170-7.
- Goldman MP, Weiss RA. Treatment of poikiloderma of Civatte on the neck with an intense pulsed light source. Plast Reconstr Surg 2001;107:1376-81.
- Weiss RA, Goldman MP, Weiss MA. Treatment of poikiloderma of Civatte with an intense pulsed light source. Dermatol Surg 2000;26:823-8.
- Behroozan DS, Goldberg LH, Glaich AS, Dai T, Friedman PM. Fractional Photothermolysis for Treatment of Poikiloderma of Civatte. Dermatol Surg 2006;32:298-301.
- Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Laser Surg Med 2004;34:426-38.
- Laubach HJ, Tannous Z, Anderson RR, Manstein D. A histological evaluation of the dermal effects after fractional photothermolysis treatment. Laser Surg Med 2005;262:86.
- Blankenship CM, Alster TS. Fractional photothermolysis of residual hemangioma. Dermatol Surg 2008;34:1112-4.
- Glaich SA, Goldberg LH, Dai T, Friedman PM. Fractional photothermolysis for the treatment of telangiectatic matting: A case report. J Cosmet Laser Ther 2007;9:101-3.
- Fitzpatrick RE, Goldman MP, Satur NM, Tope WD. Pulsed carbon dioxide laser resurfacing of photoaged facial skin. Arch Dermatol 1996;132:395-402.
- Lowe NJ, Lask G, Griffin ME, Maxwell A, Lowe P, Quilada F. Skin resurfacing with the ultrapulse carbon dioxide laser. Observations on 100 patients Dermatol Surg 1995;21:1025-9.
- Del Pozo J, Pazos JM, Fonseca E. Lower lip hypertrophy secondary to port-wine stain: Combined surgical and carbon dioxide laser treatment. Dermatol Surg 2004;30:211-4.
- Hobbs ER, Bailin PL, Wheeland RG, Ratz JL. Superpulsed lasers: Minimizing thermal damage with short duration, high irradiance pulses. J Dermatol Surg Oncol 1987;13:955-64.
Port Wine Stain
PDL is the treatment of choice for treatment of PWS, for all age groups and anatomic locations, and has the best documented safety record. [1],[2] However, clearance rates vary widely (less than 20% can be completely lightened, although 70% will lighten by 50% or more, whereas 20%?30% respond poorly). [3],[4] New-generation PDL machines (described earlier) are more effective than previous PDL machines, but vessels smaller than 30?50 μm appear resistant to all kinds of PDL treatment. [5] Epidemiological studies demonstrated that treating patients with PWS earlier in their natural history results in both greater efficacy and decreased likelihood of recurrence. [6],[7] Smaller lesions (<20 cm 2 ) clear better than larger ones, irrespective of age. [8] Head and neck lesions respond better than the lesions on trunk and limbs. Lesions on distal extremities are difficult to treat than the lesions on proximal extremities. Centrofacial lesions and those lesions located in the distribution of second branch of trigeminal nerve (V2) are less responsive than those lesions in other part of the face. [8] Pink lesions are difficult to lighten than mature red lesions. Deep purple and nodular lesions do not respond well to PDL, longer wavelength (755, 800?900, 1064 nm) are more suitable. [8] The commonest side effect is hyper pigmentation followed by hypopigmentation, hypertrophic scarring, dermal and epidermal atrophy.
The PWS has a non-uniform response to laser treatment, [9] most likely because lesions are often made of different blood vessels with different depths of involvement. [10] Different blood flow rates through vessels may also affect the efficacy of laser treatment. Darker-skinned patients can have epidermal sloughing after treatment, necessitating wound care and causing pigmentary changes. [10],[11] It is also important for darker-skinned patients to wait as long as 3?6 months between sessions to allow postinflammatory hyperpigmentation, if it has occurred, to resolve. [11] The important end point of the treatment is purpura. [12]
The treatment parameters are a wavelength of 595 nm, pulse duration range 0.45?40 ms; usually 0.45?1.5 ms, spot size: 5?10 mm, DCD spray 20?30 ms, delay 20?30 ms, and fluence ranging from 4.5 to 8.0 J/cm 2 . The number of treatments per lesion varies from 2 to 12 or more at 6?8 week intervals. Clinical end points for PDL treatment include complete lightening (<25% of cases) or, most commonly, failure to lighten any further with subsequent treatment. [13]
There are various explanations as to why PDL cannot destroy all the ectatic capillaries within PWS. These include the following. [13]
1. Inadequate depth of penetration of laser light; deeper dermal capillaries may be inaccessible to PDL light, which only penetrates up to 1 mm into the skin.
2. Inadequate conduction of laser-induced heating from the centrally situated hemoglobin chromophore to the outer vessel wall in larger diameter capillaries; hence, the vessel wall is not irreversibly damaged, allowing repair and regeneration.
3. Inadequate blood volume, hence, hemoglobin chromophore in small diameter capillaries less than 50 μm in diameter.
4. Inadequate fluence entering the capillary limits vessel wall damage.
Options for Treatment Resistant PWS [13]
1. Further treatment with the PDL using longer pulse durations ranging from 2 to 40 ms that, in theory, may treat larger diameter capillaries resistant to treatment at shorter pulse durations
2. Using pulse stacking (two to three consecutive pulses applied immediately after the preceding pulse to the same spot [14] ) may produce further lightening of PWS. The theory is that cumulative gentle heating may produce overall greater capillary heating and, hence, more efficient capillary wall damage than that achievable by a single high-energy short duration purpura-inducing pulse of laser light.
3. For PDL-resistant PWS with a purple or blue tinge, with or without thicker overlying skin and/or nodularity (because of persisting deeper and wider diameter capillaries), treatment with a more deeply penetrating, longer variable pulse width lasers such as the millisecond pulsed 1064-nm Nd:YAG has been shown to be beneficial. [15],[16]
4. Nd:YAG lasers with variable spot sizes (3, 5, 7 and 10 mm), highly variable pulse duration (0.1?300 ms) and high fluences (up to 300 J/cm 2 ) has also been used to treat PWS. There is only minimal oxyhemoglobin absorption at 1064 nm wavelength, therefore, significant non-specific tissue destruction may be produced. Scarring, textural and pigmentary changes are common. Nd:YAG laser emitting pulses in the 3?15-ms region and used at the threshold fluence for subtle, immediate purpura (i.e., at 1 minimal purpuric dose (MPD)), is safe and as effective as PDL for treating PWS lesions. [16] Histologically, Nd:YAG laser targets PWS vessels much deeper than PDL. [16] Caution is needed when using Nd:YAG laser to treat PWSs, because skin response changes rapidly at fluences greater than MPD, and MPD varies widely among different PWS lesions. [16] Using fluences higher than the minimal purpuric threshold can produce methemoglobin, which has a much higher absorption than hemoglobin or oxyhemoglobin and can lead to increased scattering. [16] Nd:YAG lasers use should be limited to nodular, hypertrophic lesions, and PDL-resistant PWS.
5. IPL is a useful alternative (to PDL), adjunctive or primary treatment (in deeper component) of PWS. [17],[18] IPL is useful in deep purple and nodular lesions. [8] These lesions have a deeper component that cannot be reached by the PDL, but can be reached by IPL or Nd:YAG. All IPL systems are not equally effective. Φzdemir et al., [19] reported moderate improvement (50%-75%) of PWS in 47% of the patients, who were treated with IPL. The optimal parameters are 515, 550, and 570 nm cut-off filter, single or multiple (2, 3) pulses, pulse width (2-10 ms) and a pulse delay of 10-20 ms. IPL treatment with a wider spectrum of wavelengths, with high fluences, and greater cumulative capillary heating with the multipulse mode may result in further destruction of residual variable diameter and deeper ectatic dermal capillaries with resultant further PWS lightening. There is clinical evidence to support this with one study showing that 50% of PDL-resistant PWS could be lightened by up to 50% with subsequent IPL treatment. [20] As clinical prediction of lightening is impossible, test treatments are advisable before embarking on this treatment course as IPL at high energy does have a significantly less favorable side-effect profile than PDL. Comparison of PDL with IPL in a randomized control study concluded that PDL has a better efficacy and higher patient preference. [21]
6. Continuous wave CO 2 laser had been used with good results for the treatment of nodules and hypertrophy in PWS. Lanigen et al, [22] published a case series of 29 patients with refractory PWS treated with CO 2 laser. Good results were seen in 74% of the patients, but scarring and depigmentation were seen in several cases. [22] Del Pozo and Fonseca published a case series of 20 patients with PWS nodules in adults treated with CO 2 laser. [23] Lesions improved by 75% with resolution of nodules and hypertrophy and the texture improvement was also noted. [23] CO 2 laser improved the contour abnormalities associated with the hypertrophic and nodular lesions, the deep violaceus color of the lesions were lightened to a light red color. [24] CO 2 laser resurfacing for PWS should primarily be targeted at adults with refractory lesions particularly to nodules and hypertrophy. [24] Early PWS in children should be treated with non-ablative lasers such as PDL.
7. The use of dual 595/1064-nm wavelength laser has been reported to improve PWS that were previously recalcitrant to ongoing PDL therapy. [1] Side effects were limited to transient erythema, edema, and mild purpura. [25] A study by Tanghetti et al., [26] also reported successful use of sequential PDL/Nd:YAG laser treatment for PWSs, showing significant improvement to resistant lesions, while using fluences lower than those used for either laser alone.
It is important to note that some PWS can recur years after treatment, despite a promising response to initial laser treatments. [27] However, most recurrences are far less visible than the original lesion and tend to develop gradually during several years. Physicians may do well to inform patients that, in some cases, laser treatment of PWSs can help manage the lesions but cannot stop their overall progression. [28] [ Table 5] gives details of types of lesion and choice of different lasers.
References
- Smit JM, Bauland CG, Wijnberg DS, Spauwen PH. Pulsed dye laser treatment, a review of indications and outcome based on published trials. Br J Plast Surg 2005;58:981-7.
- Sharma VK, Khandpur S. Efficacy of pulsed dye laser in facial port-wine stains in Indian patients. Dermatol Surg 2007;33:560-6.
- Garden JM, Bakus AD. Laser treatment of portwine stains and hemangiomas. Dermatol Clin 1997;15:373-83.
- Woo WK, Handley JM. Does fluence matter in the laser treatment of port-wine stains? Clin Exp Dermatol 2003;28:556-7.
- Kono T, Groff WF, Sakurai H. Treatment of port wine stains with the pulse dye laser. Ann Plast Surg 2006;56:460-3.
- Bernstein EF. Treatment of a resistant port wine stain with new variable pulse duration pulsed dye laser. J Cosmet Dermatol 2008;7:139-42.
- Kono T, Sakurai H, Takeuchi M, Yamaki T, Soejima K, Groff WF, et al. Treatment of resistant port-wine stains with a variable-pulse pulsed dye laser. Dermatol Surg 2007;33:951-6.
- Adamic M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPLS: Guidelines for care from the European Society for Laser Dermatology. J Cosmet Laser Ther 2007;9:113-24.
- Cantatore JL, Kriegel DA. Laser surgery: An approach to the pediatric patient. J Am Acad Dermatol 2004;50:165-84.
- Stratigos AJ, Dover JS, Arndt KA. Laser therapy. In: Bolognia JL, Jorizzo JL, Rapini RP, Horn TD, Mascaro JM, Saurat JH, et al, editors. Dermatology. London: Mosby; 2003. p. 2153-75.
- Mariwalla K, Dover JS. The use of lasers in the pediatric population. Skin Therapy Lett 2005;10:7-9.
- Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol 1990;23:1142-8.
- Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol 2007;57:677-82.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg 2004;30:163-7.
- Groot D, Rao J, Johnston P, Nakatsui T. Algorithm for using a long-pulsed Nd:Yag laser in the treatment of deep cutaneous vascular lesions. Dermatol Surg 2003;29:35-42.
- Yang MU, Yaroslavsky AN, Farinelli WA, Flotte TJ, Rius-Diaz F, Tsao SS, et al. Long-pulsed neodymium: Yttrium-aluminum-garnet laser treatment for port-wine stains. J Am Acad Dermatol 2005;52:480-90.
- Faurschou A, Togsverd-Bo K, Zachariae C, Haedersdal M. Pulsed dye laser vs intense pulsed light for port-wine stains: A randomized side-by-side trial with blinded response evaluation. Br J Dermatol 2009;160:359-64.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther 1999;1:95-100.
- Φzdemir M, Engin B, MevlitoluπI. Treatment of facial port-wine stains with intense pulsed light: a prospective study. J Cosmet Dermatol 2008;7:127-31.
- Bjerring P, Christiansen K, Troilius A. Intense pulsed light source for the treatment of dye laser resistant port wine stains. J Cosmet Laser Ther 2003;5:7-13.
- Faurschou A, Togsverd-Bo K, Zachariae C, Hζdersdal M. Pulsed dye laser vs. intense pulsed light for port-wine stains: a randomized side-by-side trial with blinded response evaluation. Br J Dermatol 2009;160:359-64.
- Lanigen SW, Cotterill JA. The treatment of port wine stains with the carbon dioxide laser. Br J Dermatol 1990;123:229-35.
- del Pozo J, Fonseca E. Port-wine stain nodules in the adult: Report of 20 cases treated by CO 2 laser vaporization. Dermatol Surg 2001;27:699-702.
- Tierney EP, Hanke CW. Treatment of nodules associated with port wine stains with CO 2 laser: Case series and review of the literature. J Drug Dermatol 2009;8:157-61.
- Alster TS, Tanzi EL. Combined 595-nm and 1,064-nm laser irradiation of recalcitrant and hypertrophic port-wine stains in children and adults. Dermatol Surg 2009;35:914-8.
- Tanghetti EA, Sherr E, Sierra R, Mirkov M. Sequential 595 nm, 1064 nm laser treatment for blebbed portwine stains and leg veins. Lasers Surg Med 2005;36:74
- Cantatore JL, Kriegel DA. Laser surgery: An approach to the pediatric patient. J Am Acad Dermatol 2004;50:165-84.
- Orten SS, Waner M, Flock S, Roberson PK, Kincannon J. Port-wine stains. An assessment of 5 years of treatment. Arch Otolaryngol Head Neck Surg 1996;122:1174-9.
Hemangioma
Laser treatment of hemangioma remains controversial
Criteria for consideration in the use of laser therapy for hemangiomas are as follows:
- Potential for functional impairment.
- Risk of ulceration, rapid enlargement, recurrent trauma, moist area
- Cosmetic disfigurement.
- Residual telangiectasia after complete involution.
PDL has the best documented safety record. [1] No age restrictions are placed when considering the PDL treatment, and even premature infants have safely undergone the therapy. [2] However, the possibility of spontaneous resolution, with or without steroid administration should be taken in to account while planning laser treatment. Beneficial effects are limited to the superficial portions of hemangioma, as PDL is limited by its depth of vascular injury (1?2 mm). [3],[4],[5],[6]
Treatment with PDL will not reduce the growth of deeper component of hemangioma. It is difficult to predict the presence of deeper component in a hemangioma, as deeper component may still grow despite successfully treating the superficial component. Evidence for effectiveness of PDL on deeper component of hemangioma is not well established. Lesions thicker than 3 mm may not respond well to PDL. [7] PDL are currently used for thin, superficial lesions, ulcerated hemangioma, residual erythema, and telangiectasias. [8],[9] optimal parameters for PDL are 585?595 nm, fluence of 5?7.5 J/cm 2 , pulse duration: 300?450 μs, spot size 5?7 mm and concomitant cooling device. [10]
For the ulcerated lesions, PDL is effective in treating pain, decreasing infection and reduces the bleeding by assisting the epithelialization of the ulcer. [11] Study by Batta et al., [12] showed no significant benefit in early PDL treatment of uncomplicated hemangioma, although treated lesions had a higher risk of atrophy and hypopigmentation. The complications of PDL are atrophic scarring, ulceration, hemorrhage, pain and residual scarring. [13] Long pulsed PDL has been reported to be safer and more effective than PDL for childhood hemangioma. [14]
For the deep components of hemangiomas, use of longer-wavelength, deeper-penetrating lasers, such as the Nd:YAG laser, have been tried with variable results, this laser carries the risk of scarring, blistering, crusting, pigmentation and textural abnormalities. [7],[15],[16] These complications are due to deep thermal injury from intensely penetrating near infrared light. Nd:YAG laser has a narrow band of safety and efficacy. [10] Ulcerated lesions are painful and require local anesthesia (1% lignocaine). Superficial ectatic blood vessels of incompletely regressed hemangioma can be treated with PDL. Lesions involving larger areas need topical, local or general anesthesia.
Fractional laser photothermolysis has been reported to be effective in patients presenting with residual hemangiomas or skin redundancy. [17] Angermeier reported 75%?100% clearance rate in patients with Centro facial hemangioma treated with IPL. [18] Greater pain levels and a high number of complications limits the use of IPL in hemangioma. [19]
Currently, there are no optimal laser systems for hemangioma treatment. [9] Various options should be considered carefully, remembering that laser treatment, especially of deep or complicated hemangiomas, may not lead to improvement and that many dermatologists prefer watchful waiting to aggressive treatment. [10]
References
- Garden JM, Bakus AD. Laser treatment of portwine stains and hemangiomas. Dermatol Clin 1997;15:373-83.
- Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol 1991;127:202-5.
- Geronemus RG. Pulsed dye laser treatment of vascular lesions in children. J Dermatol Surg Oncol 1993;19:303-10.
- Garden JM, Bakus AD, Paller AS. Treatment of cutaneous hemangiomas by the flashlamp-pumped pulsed dye laser: Prospective analysis. J Pediatr 1992;120:555-60.
- Poetke M, Philipp C, Berlien HP. Ten years of laser treatment of hemangiomas and vascular malformations: Techniques and results. In: Berlien HP, Schmittenbecher PP, editors. Laser Surgery in Children. Berlin, Germany: Springer-Verlag; 1997. p. 82-91.
- Poetke M, Philipp C, Berlien HP. Flashlamp-pumped pulsed dye laser for hemangiomas in infancy: Treatment of superficial vs mixed hemangiomas. Arch Dermatol 2000;136:628-32.
- Stratigos AJ, Dover JS, Arndt KA. Laser therapy. In: Bolognia JL, Jorizzo JL, Rapini RP, Horn TD, Mascaro JM, Saurat JH, editors. Dermatology. London: Mosby; 2003. p. 2153-75.
- Bruckner AL, Frieden IJ. Hemangiomas of infancy. J Am Acad Dermatol 2003;48:477-493.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med 1999;341:173-81.
- Galeckas KJ. Update on lasers and light devices for the treatment of vascular lesions. Semin Cutan Med Surg 2008;27:276-84.
- David LR, Malek MM, Argenta LC. Efficacy of pulsed dye laser therapy for the treatment of ulcerated hemangiomas: A review of 78 patients. Br J Plast Surg 2003;56:317-27.
- Batta K, Goodyear HM, Moss C, Williams HC, Hiller L, Waters R. Randomized controlled study of early pulsed dye laser treatment of uncomplicated childhood hemangiomas: Results of a 1-year analysis. Lancet 2002;360:521-7.
- Witman PM, Wagner AM, Scherer K, Waner M, Frieden IJ. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med 2006;38:116-23.
- Kono T, Sakurai H, Groff WF, Chan HH, Takeuchi M, Yamaki T, et al. Comparison study of a traditional pulsed dye laser versus a long-pulsed dye laser in the treatment of early childhood hemangiomas. Lasers Surg Med 2006;38:112-5.
- Willey A, Anderson RR, Azpiazu JL, Bakus AD, Barlow RJ, Dover JS, et al. Complications of laser dermatologic surgery. Lasers Surg Med 2006;38:1-15.
- Achauer BM, Celikoz B, VanderKam VM. Intralesional bare fiber laser treatment of hemangioma of infancy. Plast Reconstr Surg 1998;101:1212-7.
- Tierney EP, Kouba DJ, Hanke CW. Review of Fractional Photothermolysis: Treatment Indications and Efficacy. Dermatol Surg 2009;35:1445-61.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther 1999;1:95-100.
- Raulin C, Greve B, Grema H. IPL technology: A review. Lasers Surg Med 2003;32:78-87.
Facial Telangiectasia
The telangiectatic appearance is often considered cosmetically disfiguring, especially in people with fair skin type where the vessels may become rather distinct. Several lasers and light sources have been effectively used to treat the facial telangiectasia (KTP, PDL, LPDL, Nd:YAG, and IPL). PDL and IPL have been widely used for facial telangiectasia.
PDL has been used extensively and has a superior clearance rate compared to other lasers. [1],[2],[3] However, post-treatment purpura after PDL is cosmetically unacceptable. Purpura-free long pulsed PDL has shown good efficacy. [1],[2],[3] With PDL, purpura-free and purpura-level treatments may be close to be equivalent for treating fine telangiectasia, but purpura-level treatments have a distinct advantage for treating thicker telangiectasia. [3] However, some authors have noted inferior results in purpura-free treatments compared with those treatments that cause purpura. [3] Significantly, the variable-pulse pulsed-dye laser offers patients the option of effective treatment of some telangiectasia without bruising. [3] The optimal parameters for LPDL are 7?10 mm spot size, 6?20 ms pulse width, 7?12 J/cm 2 , DCD - 30/10, and two to three passes. [1]
IPL is an alternative or a supplement to the existing laser devices in treatment of facial telangiectasia. As first described by Schroeter and Neumann [4] then confirmed by Angermeier [5] and Bjerring et al, [6] the IPL is useful for the treatment of facial teleangectasias. Clementoni et al, [7] reported that 87% of patients presented a clearance of 75?100% with 1?2 IPL sessions. The optimal parameters for large veins are triple pulse mode using the 590 nm cut-off filter with pulse times of 2.4, 3.0, and 3.5 ms, delay of 30 and 25 ms and double pulse mode using the 570 nm cut-off filter with pulse times of 4.0 ms, delay of 30 ms for red fine telangiectasia, and spider lesions. [7] Perilesional erythema, blanching, or vessel clearance are the optimal treatment endpoints.
Intraindividual, randomized, controlled trial with split-face treatments and single-blind outcome evaluation comparing LPDL and IPL for the treatment of facial telangiectasia concluded that IPL and LPDL reduce facial telangiectasias by more than 75% in just three treatment sessions. [1] However, LPDL treatments resulted in superior vessel clearance than IPL treatments and the majority of patients reported that they preferred the LPDL treatments due to superior efficacy and less treatment-related pain. [1] A randomized split-face trial with blinded response evaluation comparing long-pulsed dye laser versus intense pulsed light for photodamaged skin: reported that LPDL was superior to IPL for telangiectatic lesions. [2] Both the above studies compared LPDL with IPL devices without cooling systems. It might be possible that using an IPL-system with a cooling device, the results might have resulted in a better efficacy.
Nd:YAG laser should be considered as another modality in the armamentarium of devices used to treat telangiectasias of the face. Eremia and Li [8] reported a prospective study of a variable pulse 1064 nm Nd:YAG laser, 6 mm spot size on facial telangiectasias and reported a clearance of 75% of telangiectasias at 1 month in 97% of the sites that were treated. Sarradet and colleagues demonstrated moderate to significant improvement in 80% of patients 3 months following long pulse width 1064 nm Nd:YAG treatment of telangiectasias of the face. [9] A study by Major and colleagues reported swelling and crusting that lasted for a few days in every 1 of 25 patients treated with the 1064 nm Nd:YAG laser for facial telangiectasias. [10] A small spot size (1.5 mm) Nd:YAG laser using a pulse width of 20 ms or higher reported clearing in 50?75% of facial ectasias with a single pass with minimal side effects. [11]
Every light source used for facial telangiectasia has its own limitations, PDL has been used extensively and has a superior clearance rate compared with other lasers. However, post-treatment purpura after PDL is cosmetically unacceptable. Purpura-free long pulsed PDL has shown good efficacy. However some authors have noted inferior results in purpura-free treatments compared with those treatments that cause purpura. [3] IPL has the side effect of significant erythema and edema. The Nd:YAG laser allows for treatment of a broad range of vessel diameters in most skin types. However, relatively poor absorption by hemoglobin and pigment (versus highly absorbing green yellow light) limits its usefulness. Caution should be exercised, while using the machines in darker skin patients because of the risk of post-treatment pigmentation and, hence, proper patient counseling is essential.
References
- Nymann P, Hedelund L, Hζdersdal M. Long-pulsed dye laser vs. intense pulsed light for the treatment of facial telangiectasias: A randomized controlled trial. J Eur Acad Dermatol Venereol 2010;24:143-6.
- Jψrgensen GF, Hedelund L, Hζdersdal M. Long-pulsed dye laser versus intense pulsed light for photodamaged skin: A randomized split-face trial with blinded response evaluation. Lasers Surg Med 2008;40:293-9.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: Comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg 2003;29:681-5.
- Schroeter CA, Neumann HA. An intense light source: The photoderm VL-flashlamp as a new treatment possibility for vascular skin lesions. Dermatol Surg 1998;24:743-8.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther 1999;1:95-100.
- Bjerring P, Christiansen K, Troilius A. Intense pulsed light source for treatment of facial teleangectasias. J Cosmet Laser Ther 2001;3:169-73.
- Clementoni MT, Gilardino P, Muti GF, Signorini M, Pistorale A, Morselli PG, et al. Facial teleangectasias: Our experience in treatment with IPL. Lasers Surg Med 2005;37:9-13.
- Eremia S, Li CY. Treatment of face veins with a cryogen spray variable pulse width 1064 nm Nd:YAG laser: A prospective study of 17 patients. Dermatol Surg 2002;28:244-7.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium: YAG laser treatment of facial telangiectases. Dermatol Surg 2003;29:56-8.
- Major A, Brazzini ΐB, Campolmi P, Bona P, Mavilia L, Ghersetich I, et al. Nd:YAG 1064 nm laser in the treatment of facial and leg telangiectasias. J Eur Acad Dermatol Venereol 2001;15:559-65.
- Bevin AA, Parlette EC, Domankevitz Y, Ross EV. Variable-Pulse Nd:YAG laser in the treatment of facial telangiectasias. Dermatol Surg 2006;32:7-12.
Rosacea Associated Telangiectasia and Erythema
Long pulse duration, 595 nm, PDL is safe and effective for treating rosacea with minimal side effects and no long-term complications. [1] Clark et al, [2] reported a significant reduction in both erythema and telangiectasia of rosacea in all of their patients after treatment with PDL. Nevertheless, there was accompanying immediate post-treatment purpura. They also reported pigmentation and atrophic scarring in a number of their patients. These resolved after few months. [2] Similar side effects were reported in other studies that used PDL for treatment of telangiectatic lesions. [3],[4],[5],[6] All of these studies used PDL in short pulse duration. Although reversible in the majority of cases, this may have more profound cosmetic consequences than facial telangiectasia. Lowe et al, [7] used flash-lamp PDL with good improvement of telangiectasia and erythema in almost all of their patients.
People with rosacea often have both linear vessels and diffuse redness; the use of two separate settings 40 ms for linear telangiectasia and 3 ms pulse duration for diffuse erythema enabled optimal treatment of linear vessels and diffuse erythema. [1] Purpura-free removal of cutaneous vessels has been documented using pulse-durations of 6 and 10 ms [8],[9] Longer pulse-durations result in less purpura at fluences necessary to remove most vessels as compared with much shorter pulse durations. The long-pulsed 595-nm PDL using single session subpurpuric clinical threshold was effective in 75% cases for the treatment of rosacea associated telangiectasia. [10] The drawback to purpura-free PDL is the lack of obvious visible end points and the possibility that excessive overlapping might increase the incidence of scarring. [3]
Treatment of rosacea with the PDL has been shown to improve not only the telangiectasias and erythema, but also the symptoms associated with rosacea. [7],[9],[11],[12],[13] The improvement of other signs of photodamage, such as enlarged pores or textural irregularity probably resulted from the subsequent inflammatory response to laser treatment.
The 595-nm PDL parameters are 10-mm spot size, 6-ms pulse duration, and 7 J/cm 2 with cryogen cooling (30-ms spray administered 20 ms before the laser firing). The fluence can be adjusted up or down at 0.5 J/cm 2 intervals to achieve a transient purpura lasting for less than 3 s that subsequently resolves.
IPL is a safe and effective treatment of erythematotelangiectatic (ET) rosacea. It significantly reduces erythema and telangiectasia with minimal unwanted effects. [14] In a pilot study by Mark et al., [15] a 30% decrease in blood flow, a 29% decrease in area of telangiectasia, and a 21% decrease in erythema intensity were found after five IPL sessions. Taub [16] treated patients with rosacea with one to seven IPL sessions. Eighty-three percent of patients had reduced redness, 75% noted reduced flushing and improved skin texture, and 64% noted fewer acneiform breakouts. Schroeter et al., [17] have demonstrated that IPL can be effectively used for long-term clearance of telangiectasia associated with rosacea. Seventy-eight percent of those lesions responded to treatment and less than 1% recurrence was observed over a 3-year follow-up period. IPL parameters are spectrum ranging from 515 to 1200 nm with different pulse durations between 2 and 6 ms, single or multiple pulse mode with 15-ms pulse delay.
A randomized, controlled, single-blind, split-face trial comparing a series of non-purpuragenic PDL treatment with IPL treatment and untreated control in ET rosacea resulted in the improvement in cutaneous erythema, telangiectasias, and patient-reported symptoms. IPL and PDL performed with similar efficacy and safety, and both modalities seem to be reasonable choices for the treatment of ET rosacea. [18]
Refernces
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med 2008;40:233-9.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci 2002;17:26-33.
- Travelute Ammirati C, Carniol PJ, Hruza GJ. Laser treatment of facial vascular lesions. Facial Plast Surg 2001;17:193-201.
- Ross M, Watcher MA, Goodman MM. Comparison of the flash lamp pulsed dye laser with the argon tunable dye laser with robotized hand piece for facial telangiectasia. Lasers Surg Med 1993;13:374-8.
- Bernstein EF, Lee J, Lowery J, Brown DB, Geronemus R, Lask G, et al. Treatment of spider veins with the 595 nm pulsed-dye laser. J Am Acad Dermatol 1998;39:746-50.
- Broska P, Martinho E, Goodman MM. Comparison of the argon tunable dye laser with the flashlamp pulsed dye laser in treatment of facial telangiectasia. J Dermatol Surg Oncol 1994;20:749-53.
- Lowe NJ, Behr KL, Fitzpatrick R, Goldman M, Ruiz-Esparza J. Flash lamp pumped dye laser for rosacea-associated telangiectasia and erythema. J Dermatol Surg Oncol 1991;17:522-5.
- Bernstein EF. The new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser improves the appearance of photodamaged skin. Lasers Surg Med 2007;39:157-63.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology and quality of life. J Am Acad Dermatol 2004;51:592-9.
- Jasim ZF, Woo WK, Handley JM. Long-Pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg 2004;30:37-40.
- Tan ST, Bialostocki A, Armstrong JR. Pulsed dye laser therapy for rosacea. Br J Plast Surg 2004;57:303-10.
- Orenstein A, Nelson JS. Treatment of facial vascular lesions with a 100-μm spot 577-nm pulsed continuous wave laser. Ann Plast Surg 1989;23:310-6.
- Lonne-Rahm S, Nordlind K, Edstrom DW, Ros AM, Berg M. Laser treatment of rosacea: A pathoetiological study. Arch Dermatol 2004;140:1345-9.
- Papageorgiou P, Clayton W, Norwood S, Chopra S, Rustin M. Treatment of rosacea with intense pulsed light: Significant improvement and long-lasting results Br J Dermatol 2008;159:628-32.
- Mark KA, Sparacio RM, Voigt A, Marenus K, Sarnoff DS.. Objective and quantitative improvement of rosacea-associated erythema after intense pulsed light treatment. Dermatol Surg 2003;29:600-4.
- Taub AF. Treatment of rosacea with intense pulsed light. J Drugs Dermatol 2003;2:254-9.
- Schroeter CA, Haaf-von Below S, Neuman HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg 2005;31:1285-9.
- Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of non-purpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg 2009;35:920-8.
Poikiloderma of Civatte
Treatment of poikiloderma of Civatte by selective photothermolysis requires wavelengths that are absorbed concurrently by two different chromophores, namely hemoglobin and melanin. [1] The ectatic vessels are around 0.1 mm in diameter and reside in the papillary dermis. [1] They contain mostly oxyhemoglobin that absorbs light at 418, 542, and 577 nm. [1] Melanin, concentrated in the overlying basal epidermis, absorbs light continuously over a broad spectrum. Absorption is highest at lower wavelengths and decreases steadily over the optical range of 250-1200 nm. [2] Several treatment modalities, including argon lasers, potassium titanyl phosphate (KTP) lasers, pulsed dye lasers, and intense pulsed light devices, have been used to treat this condition. [3] Complete clearing is difficult to achieve. [3] Depending on the modality used, adverse effects such as scarring with irregular hypopigmentation, post-inflammatory hyperpigmentation, post-treatment purpura, mottled appearance, crusting and erythema have been reported. [4],[5],[6],[7],[8],[9],[10],[11] Multiple sessions with these treatments are usually necessary to obtain optimal clearing. [6],[8] While vascular ectasias should respond to laser treatment, epidermal atrophy and hypermelanosis associated with poikiloderma of civatte may not respond. [1] Great care is needed when using PDL. Pigmentary changes are late, but significant complications. [12],[13]
The newer generation pulsed dye lasers with a wavelength of 595 nm and a pulse duration of 1.5 ms in conjunction with cryogen spray cooling produce optimal clearing of poikiloderma-associated telangiectasias and pigmentation with minimal reticulation and purpura production. Optimal outcomes are achieved using a 10 mm spot size with lower fluences. [1] More research is needed to define an optimal pulse duration. Fractional photothermolysis has been reported to be effective in removing the telangiectatic component. [14] Uncomplicated treatment of non-facial skin areas with a paucity of pilosebaceous glands, such as the neck, is an exciting consideration, particularly given the historical avoidance of these anatomic locations given the risks of scarring and dyschromia with other ablative techniques. [14] Further larger, long-term studies are needed to better elucidate the efficacy and safety of fractional photothermolysis treatment for poikiloderma of Civatte and to optimize treatment parameters.
With the problems encountered using lasers, and the fact that few treat vascular and pigment abnormalities simultaneously, the intense pulsed light source has been utilized with good results. With IPL, the target is vascular, epidermal, and dermal melanin due to the broad band of wavelengths. The near-infrared component of IPL appears to improve skin texture, with a reversal of the cutaneous atrophy typically associated with poikiloderma of Civatte. [1] A retrospective study reported a significant reduction of 75%-100% of poikiloderma changes observed in 111 of 135 patients (82%) after typically three treatments using 515 nm filter, single pulse 3 ms/double pulse of 2.4 and 4 ms (10 ms delay between the pulses), and fluences from 25 to 42 J/cm 2 . [1] Adverse effects were minimal and transient, including erythema, rare short-term purpura, and rare crusting. [1]
Special care must be taken when using lasers or light sources to treat poikiloderma of the neck. The skin of the neck is thin and there is greater risk of atrophy and hypopigmentation with laser treatment. Optimal results can be achieved in the treatment of poikiloderma of Civatte with both the intense pulsed light source and pulsed dye lasers. Adverse effects are minimized with the use of larger diameter spot sizes, lower fluences, non-overlapping pulses, and repetitive treatments. Patients may expect improvement in poikiloderma-associated telangiectasias and pigmentation, as well as the additional benefit of a yet uncharacterized improvement in skin texture.
References
- Weiss RA, Goldman MP, Weiss MA. Treatment of poikiloderma of Civatte with an intense pulsed light source. Dermatol Surg 2000;26:823-7.
- Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol 1981;77:13-9.
- Tierney EP, Kouba DJ, Hanke CW. Review of fractional photothermolysis: Treatment indications and efficacy. Dermatol Surg 2009;35:1445-61.
- Goldman L, Bauman WE. Laser test treatment for postsolar poikiloderma. Arch Dermatol 1984;120:578-9.
- Oldbricht SM, Stern RS, Tang SV, Noe JM, Arndt KA. Complications of cutaneous laser surgery: A survey. Arch Dermatol 1987;123:345-9.
- Batta K, Hinson C, Cotterill JA, Foulds IS. Treatment of poikiloderma of Civatte with potassium titanyl phosphate (KTP) laser. Br J Dermatol 1999;140:1191-2.
- Wheeland RG, Applebaum J. Flashlamp pumped pulsed dye laser therapy for poikiloderma of Civatte. J Dermatol Surg Oncol 1990;16:12-6.
- Haywood R, Monk BE. Treatment of poikiloderma of Civatte with the pulsed dye laser: A series of seven cases. J Cutan Laser Ther 1999;1:45-8.
- Geronemus RG, Kauvar AN. Successful treatment of poikiloderma of Civatte utilizing the pulsed dye laser. Laser Surg Med 1999;112:62.
- McCoy S, Grevelilink JM. Results of patients treated for poikiloderma of Civatte with the flashlamp pumped dye laser (at 585 nm). Laser Surg Med 1998;102:44-5.
- Goldman MP, Weiss RA. Treatment of poikiloderma of Civatte on the neck with an intense pulsed light source. Plastic Recon Surg 2000;106:1376-81.
- Meijs MM, Blok FA, de Rie MA. Treatment of poikiloderma of Civatte with the pulsed dye laser: a series of patients with severe depigmentation. J Eur Acad Dermatol Venereol 2006;20:1248 -51.
- Geronemus R. Poikiloderma of Civette. Arch Dermatol 1990;126:547-8.
- Behroozan DS, Goldberg LH, Glaich AS, Dai T, Friedman PM. Fractional photothermolysis for treatment of poikiloderma of civatte. Dermatol Surg 2006;32:298-301.
Pyogenic Granuloma
Various laser devices have been successfully used to treat pyogenic granulomas, including the 585- and 595-nm PDLs, the 1064-nm Nd:YAG, and the CO 2 laser. PDL is a safe, effective, and reasonable alternative to conventional therapy. It is safe for the treatment of small pyogenic granulomas in children and should be considered as a treatment option. [1],[2] Tay and colleagues reported 91% clearance with 75% of them requiring two or more treatments. Lesions that were raised more than 5 mm from the surface did not respond to the PDL. [1] Owing to its limited penetration depth, PDL can only be used to treat small pyogenic granulomas. Gonzalez and colleagues reported that 16 out of 18 patients had symptomatic and clinical clearing of the lesions with excellent cosmetic results and no postoperative complications, persistent pigmentary changes or scarring were observed. The procedure required no anesthesia, and postoperative care was limited to the application of a topical antibiotic ointment. [2] Coagulation, retraction or shrinkage of the lesion is the end point.
The CW carbon dioxide (CO 2 ) laser has proved to be an effective treatment option. [3] Its use permits rapid, minimally invasive surgical treatment, larger lesions can be effectively treated, convalescence takes less time, can treat difficult to access regions and in those lesions which bleeds heavily, but the non-specific coagulation may lead to scars. [4] The combined continuous-wave/pulsed CO 2 laser has been reported to be effective for pyogenic granuloma, 98% of patients responded within one session without recurrence. [4] The laser was first used in continuous mode (power, 15 W; focused hand piece with variable spot size of 1.5?3.0 mm) and then in pulsed mode (pulse length, 0.6?0.9 ms; fluence, 500 mJ/pulse), with the same hand piece. [4] CO 2 laser is easy to use, yields low recurrence rates, and is well tolerated by most patients. [4]
The other lasers systems that have been tried are, Nd:YAG and IPL, these lasers require multiple treatment sessions. [5] There are reports about the induction of pyogenic granuloma due to the use of the PDL, Nd:YAG laser, and argon laser. [6],[7],[8] Any laser therapy should only be done if it is certain that the lesion is benign. If there is any doubt, conventional surgical methods or laser treatment should be used only with histological confirmation.
Evidence: Level B
REFERENCES
- Tay YK, Weston WL, Morelli JG. Treatment of pyogenic granuloma in children with the flashlamp-pumped pulsed dye laser. Pediatrics 1997;99:368-70.
- Gonzαlez S, Vibhagool C, Falo LD Jr, Momtaz KT, Grevelink J, Gonzαlez E. Treatment of pyogenic granulomas with the 585 nm pulsed dye laser. J Am Acad Dermatol 1996;35:428-31.
- Raulin C, Petzoldt D, Werner S. Granuloma pyogenicum --removal with the CO2 laser. Hautarzt 1997;48:402-5.
- Raulin C, Greve B, Hammes S. The combined continuous-wave/pulsed carbon dioxide laser for treatment of pyogenic granuloma. Arch Dermatol 2002;138:33-7.
- Adamic M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPLS: Guidelines for care from the European Society for Laser Dermatology. J Cosmet Laser Ther 2007;9:113-24.
- Abd-el-Raheen TA, Hohenleutner U, Landthaler M. Granuloma pyogenicum as a complication of flashlamp-pumped pulsed dye laser. Dermatology 1994;189:283-5.
- Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, Baumler W, Landthaler M. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains: A prospective study. Br J Dermatol 1996;134:475-80.
- De Rooij MJ, Neumann HA. Granuloma telangiectaticum after argon laser therapy of a spider nevus. Dermatol Surg 1995;21:356-7.
Venous Lakes
Venous lakes (VLs) are common benign venous ectasias in the upper dermis. They are treated to improve cosmesis and occasionally to prevent bleeding. Numerous methods have been used, such as cryotherapy, infrared coagulation, and various types of lasers. They are variable in their success and all can be complicated by scarring. [1]
The long-pulsed Nd:YAG laser is effective treatment for venous lakes of the lip and cheeks, 94% clearance has been reported with single treatment with either a 3-mm spot at 250 J/cm 2 and 55 ms or a 5-mm spot at 140?180 J/cm 2 depending on the size of lesion. [2] Clinical end points are characterized by hardening of the lesion, central blackening, minimal whitening of the periphery, and in most cases, an audible popping sound. [2] The PDL can be used to treat venous lakes with multiple overlapping pulses. [3] The limited success with PDL laser could be attributed to insufficient thermal energy being generated to close all the blood vessels permanently. [1]
Carbon dioxide laser is a good and safe method. Resolution of lesions has been reported with single session of treatment using a continuous and defocused mode, a power density of 5 W/cm 2 , in the first pass, and a continuous focused mode with the same power density in the second pass. [4] Multi wavelength laser (595 nm; 1064 nm) has been reported as safe, fast, and effective option with the following parameters 595-nm pulsed-dye laser at 20 ms and 10 J/cm 2 , followed by 1064-nm neodymium-doped yttrium aluminum garnet laser at 20 ms and 70 J/cm 2 . [1]
References
- Roncero M, Canueto J, Blanco S, Unamuno P, Boixeda P. Multiwavelength laser treatment of venous lakes. Dermatol Surg 2009;35:1942-6.
- Bekhor PS. Long-pulsed Nd:YAG laser treatment of venous lakes: Report of a series of 34 cases. Dermatol Surg 2006;32:1151-4.
- Cheung ST, Lanigan SW. Evaluation of the treatment of venous lakes with the 595-nm pulsed-dye laser: a case series. Clin Exp Dermatol 2007;32:148-50.
- Pozo JD, Peρa C, Silva JC, Jose Goday JJ, Fonseca E. Venous Lakes: A report of 32 cases treated by carbon dioxide laser vaporization. Dermatol Surg 2003;29:308-10.
Cherry Angioma
Using a pulsed dye laser is the preferred method of treatment for cherry angioma among lasers. [1] The other laser systems that have been tried are Nd:YAG and IPL. [1] However, it should be noted that cherry angioma can be treated with simpler treatments such as cryosurgery, and radiofrequency, which are cheaper and easily available.
Reference
- Adamic M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPLS: Guidelines for care from the European Society for Laser Dermatology. J Cosmet Laser Ther 2007;9:113 -124.
Leg Veins
Leg veins are difficult to treat by laser, the reasons are: increased hydrostatic pressure on the lower extremities, anatomy of lower extremity blood vessels (leg veins have a thick surrounding adventitial tissue and increased basal lamina), occasional association with venous disorders and reduced amount of oxyhemoglobin. [1] To thermocoagulate the deeper vessels of greater diameter, laser systems should be able to deliver very high energy pulses through large spot sizes to enhance scattering into dermis.
Lasers should be considered as an alternative to sclerotherapy in patients with needle phobia, who do not tolerate sclerotherapy, who fail to respond to sclerotherapy, who had developed adverse effects from sclerotherapy, and who are prone to telangiectatic matting. [1]
Lasers enable treatment of the following: [1]
- Spider veins: 0.2?2 mm red and blue vascular ectasias, often associated with large reticular veins.
- Reticular veins: non-bulging subcutaneous veins ranging up to 5 mm in diameter.
- Telangiectasias: 0.2-1 mm, residing about 300 μm below the skin surface, from dark blue to bright red.
Nd:YAG laser systems are the lasers of choice for leg vein treatment. [1] The 3 and 5 mm spot sizes are suitable for superficial lesions such as telangiectasias, 5 or 7 mm spot sizes are needed for thicker or deeper veins. [1] Pulse durations should be selected according to the estimated vessel size of each lesion; 0.3?5 ms for thin, 10?25 ms for medium, and 25?55 ms for thick vessels. [1] Spider veins, reticular veins and leg telangiectasia require longer pulse widths of 20-50 ms or longer, since the vessels are larger (0.1-5 mm) than vessels comprising port wine stains. [2] Variable fluences (from 14 to 195 J/cm 2 ) are used. Nd:YAG lasers used with 50 ms pulses are effective in the treatment of reticular leg veins measuring 1-3 mm in diameter. [3] Overlapping of the treated area is not necessary and the pulses can be spaced 1 mm apart. Treatment with long-pulsed 1064-nm lasers is painful; therefore, cooling and either topical anesthesia or local anesthesia are necessary. In the direct-contact method, minimal pressure is placed against the skin. In some cases in which a larger reticular vein is targeted for 1064-nm laser, slight pressure may be used to minimize the total diameter of the vein to allow greater penetration and less total heat accumulation by reducing target size. This also may make the treatment slightly less painful.
Long-pulsed PDLs have been shown to clear vessels with diameters less than 0.5 mm completely. For vessels 0.5-1 mm, improvement, but not clearance, is achieved. [2] For leg vein treatment, the 1064 nm wavelength is very safe for type V skin, the 810 nm diode at super-long pulse widths of 400-1000 ms is very safe for type IV and marginal for type V skin, and the 755 nm alexandrite is limited to non-tanned type I-III skin. [4] Leg veins of <1 mm diameter respond better to IPL. [5]
The clinical end point is the darkening of the targeted vessel and loss of vessel margins. Blanching of skin should be avoided. Hyperpigmentation is common even in light-skinned individuals; this usually resolves with time. [2] A drawback to laser therapy and sclerotherapy is that the usual underlying cause of spider veins, incompetence of a large underlying vein, is not treated. Subsequently, new spider veins continue to form after treatment. Simultaneous treatment with lasers of longer wavelengths and pulse durations for larger veins in combination with lasers of shorter wavelengths and pulse durations for the smaller veins may provide comparable outcomes to treating patches of heterogeneous vessels by sclerotherapy. [1]
References
- Adamic M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPLS: Guidelines for care from the European Society for Laser Dermatology. J Cosmet Laser Ther 2007;9:113-24.
- Schmults CD. Laser treatment of vascular lesions. Dermatol Clin 2005;23:745-55.
- Omura NE, Dover JS, Arndt KA, Kauvar AN. Treatment of reticular leg veins with a 1064 nm long-pulsed Nd:YAG laser. J Am Acad Dermatol 2003;48:76-81.
- Eremia S, Li C, Umar SH. A side-by-side comparative study of 1064 nm Nd:YAG, 810 nm diode and 755 nm alexandrite lasers for treatment of 0.3-3mm leg veins. Dermatol Surg 2002;28:224-30.
- Fodor L, Ramon Y, Fodor A, Carmi N, Peled IJ, Ullmann Y. A side-by-side prospective study of intense pulsed light and Nd:YAG laser treatment for vascular lesions. Ann Plast Surg 2006;56:164-70.
Complications of Laser Treatment for Vascular Lesions
1. Pain: Pain is frequent and may be a marker for the subsequent adverse effect. Pain can be reduced with appropriate anesthetic techniques as mentioned earlier.
2. Edema: Some amount of erythema and edema is expected at and around the treated site immediately. Periorbital and neck are more prone for edema. Usually the edema subsides by 5?7 days. Appropriate cooling during the procedure and after the treatment reduces the edema.
3. Bleeding: Bleeding occurs due to inappropriate parameters (high fluence and short pulse duration). Proper parameter selection reduces the incidence of bleeding.
4. Purpura: Purpura is seen immediately after the treatment and usually fades by 7-10 days. No specific treatment is required.
5. Blistering and discoloration are seen rarely. They are usually caused by over treatment. Starting with a test patch, appropriate parameter selection and proper cooling reduces blistering. Post-inflammatory hypo/hyper pigmentation may result following blistering. Hyperpigmentation is seen more commonly in darker skin types. It usually fades in 3?6 months time. Topical bleaching creams such as hydroquinone speed up the recovery time. Hydroquinone topical application 2 weeks prior to the laser session reduces the incidence of hyperpigmentation. Hypopigmentation usually gets corrected in 3?6 months time.
6. Skin texture changes are caused by over treatment (due to excessive fluences or pulse stacking)
7. Scarring: Usually due to over treatment or following disruption of skin surface. Following the pre- and post-laser treatment care strictly reduces the chance of scarring.
8. Reactivation of herpes: Prophylactic oral virostatic therapy is recommended for patients with history of herpes, particularly while treating lesions on face.
9. Infection: Infection is uncommon; edema, erythema, pain, oozing, crusting and fever indicates infection. Topical and systemic antibiotics should be used.
10. Nonresponders: some lesions do not respond despite the adequate treatment. This possibility should be informed to patients prior to treatment and proper pretreatment counseling is recommended in all cases.
Prelaser Treatment Care
- Detailed pretreatment education should be done to the patients with information sheets and brochures.
- Informed consent is a must in all cases (see Appendix 1).[SUPPORTING:1]
- Photography, preoperatively at each session is required in all case.
- Patients medical history should be analyzed for any contraindications for laser treatment.
- Topical bleaching creams for dark skinned individuals at least 2 weeks prior to the laser treatment.
- A broad-spectrum sunscreen at least 4 weeks prior to the treatment session.
- Test treatment on a representative area may be performed
- Adequate eye protection goggles are necessary for all persons in the laser room.
- For treating lesions on the eye lids, corneal shields are a must to protect the eye.
- Patients data should be recorded in a separate log which includes, fluence used, site of lesion, wavelength used, total number of pulses and any untoward response during the treatment.
Postlaser Treatment Care
- Adequate cooling post laser treatment with ice packs on larger areas.
- Patients should be asked to sleep with extra pillow to encourage the gravitational removal of inflammatory fluid, for treating lesions around the eye.
- Patients should be instructed to use a broad-spectrum sunscreen post laser treatment.
- Patients should be instructed not to pick or scratch the treated sites.
- Topical antibiotics in a cream or ointment base are prescribed for the treated site to prevent infection.
- Patient is advised to use mild non-irritating soaps on the treated site.
- Mild cosmetics may be used after laser, except in cases of blistering.
- Patients should avoid swimming and contact sports during healing time.
Summary
There are a variety of options available for treating vascular lesions. Different laser systems are used, often at different fluences, pulse widths and spot sizes, to treat the same vascular lesions. The physician should choose the proper treatment parameters for each laser system, vascular lesion and body location. In view of the variabilities in the laser specifications and individual responses, a test treatment on a small, but representative site is recommended. The treatment protocol must be based individually on each patient′s clinical examination, skin type, history and tissue response. The final judgment lies in the hands of the physician [Table - 5],[Table - 6].


Fulltext Views
26,814
PDF downloads
4,364





