Translate this page into:
Leprosy reactions: New knowledge on pathophysiology, diagnosis, treatment and prevention
Corresponding authors: Dr. Tarun Narang, Department of Dermatology, Venereology and Leprology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. narangtarun@yahoo.co.in, Dr. Sunil Dogra, Department of Dermatology, Venereology and Leprology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. sundogra@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mehta H, Jain S, Narang T, Chhabra S, Dogra S. Leprosy reactions: New knowledge on pathophysiology, diagnosis, treatment and prevention. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_915_2024
Abstract
Leprosy, or Hansen’s disease, caused by Mycobacterium leprae and Mycobacterium lepromatosis, is a chronic granulomatous infectious disease. Leprosy reactions, characterised by neurocutaneous inflammation, complicate the disease’s indolent course, leading to significant morbidity. However, limited knowledge of reaction pathophysiology stems from a lack of experimental models and the abrupt onset of reactional episodes, posing challenges in delineating initial pathogenic steps. In type 1 reactions, ongoing studies explore the roles of interferon-gamma which results in increased interleukin (IL)-15 and autophagy. Leprosy reactions also exhibit an increase in T helper 17 (Th17) and a decrease in T-regulatory cell (Treg) populations, resulting in diminished tumour growth factor-beta and heightened IL-6 and IL-21 production. Exploring the pathogenesis of erythema nodosum leprosum (ENL) reveals insights into neutrophils, Toll-like receptor 9, B-cells, myeloid-derived suppressor cells, IL-10 pathway and neurotrophins. Noteworthy therapeutic targets include increased expression of cyclooxygenase 2 and vascular endothelial growth factor. Early reaction diagnosis is crucial to limit neural damage, with high-resolution ultrasonography showing promise in detecting minimal nerve involvement. Therapies for ENL management, such as thalidomide, methotrexate, apremilast, minocycline and tumour necrosis factor-alpha inhibitors, hold potential. This review addresses recent advances in leprosy reaction pathogenesis and diagnostics, offering therapeutic insights and preventive strategies to mitigate their onset.
Keywords
Leprosy
Hansen disease
leprosy reactions
reversal reactions
erythema nodosum leprosum
type 1 reaction
type 2 reaction
Introduction
Leprosy reactions are acute immunologically driven episodes in the chronic course of leprosy that result in significant functional morbidity. These may occur prior to, during or following the completion of multidrug therapy (MDT), with a reported lifetime prevalence of nearly 50% among leprosy patients.1,2 Two major types of leprosy reactions include reversal reactions (RRs) or type 1 reaction (T1R) and erythema nodosum leprosum (ENL) or type 2 reaction (T2R). RRs are seen in up to one-third of patients in the borderline spectrum of leprosy.3 RRs are type IV hypersensitivity reactions due to an increase in cell-mediated immune response (CMI) against Mycobacterium leprae (M. leprae). ENL occurs in 50% of patients with lepromatous leprosy (LL) and 10% of patients with borderline lepromatous (BL) leprosy.4 ENL can be classified based on its duration and recurrence. Acute ENL refers to an episode lasting less than six months, with treatment gradually tapered off and no recurrence while on therapy. Recurrent ENL involves a new episode occurring at least 28 days after completing treatment for a prior episode, while chronic ENL persists beyond six months, requiring continuous treatment or having treatment-free intervals shorter than 28 days. ENL is a type III hypersensitivity reaction with deposition of immune complexes, neutrophilic infiltrate and increased levels of circulating tumour necrosis factor-alpha (TNF-α). Lack of a satisfactory animal model that reproduces the whole spectrum of leprosy and reactional episodes is a major limitation in understanding the pathophysiology of the disease.
Pathophysiology
Type 1 reaction
T1R is a condition characterised by T-cell hypersensitivity against M. leprae in the borderline spectrum of leprosy. Upon M. leprae inoculation in the nasal mucosa, the immune response is initiated involving phagocytosis by antigen-presenting cells (APCs). These APCs capturing antigens from peripheral tissues migrate to lymph nodes, where they stimulate the adaptive immune response through major histocompatibility complex class II (MHC-II) molecules and two signals to naïve CD-4+ T helper (Th) cells. Interleukin (IL)-12 production by APCs promotes immune activation by differentiating CD4+ T-cells into effector Th cells. Effector Th cells release IL-2, stimulating the proliferation of Th cells and CD8+ T-cells. These activated T-cells migrate back to peripheral tissues, where CD8+ T-cells release perforin and granzymes, damaging myelin sheath and Schwann cells.3 Naïve T-cells differentiate into Th17cells due to IL-6 and tumour growth factor-beta (TGF-β) production by APCs. IL-17 from activated Th17 cells aids neutrophil recruitment. Interferon-gamma (IFN-γ) from APCs activates macrophages, leading to pro-inflammatory cytokine production (TNF-α, IL-1β, IL-6). IL-6 is thus produced by both APCs and activated macrophages and underlies the inflammatory process. Figure 1 illustrates the pathogenesis of T1R. TNF-α and IFN-γ directly stimulate nerve fibres, causing neuritis in type I reactions [Figure 2].5 This inflammatory environment, coupled with mycobacterial killing and antigenic spread, determines the progression of reactional episodes.
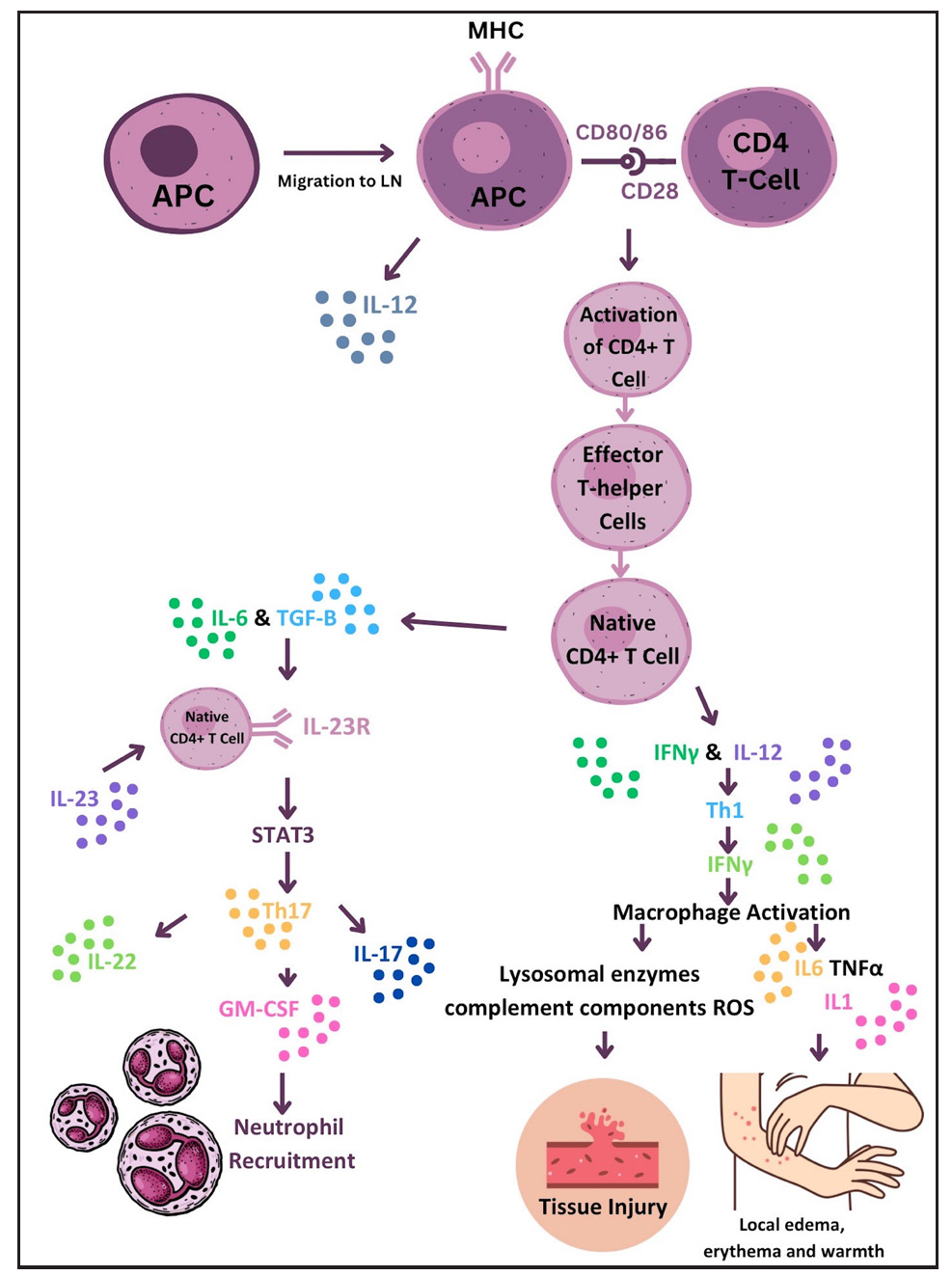
- Diagram illustrating the immune response to M. leprae in Type 1 reaction that leads to erythema, oedema and tissue injury. (APC: Antigen-Presenting Cell, CD4: Cluster of Differentiation 4, GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor, IFN: Interferon, LN: Lymph Node, MHC: Major Histocompatibility Complex, MHC II: Major Histocompatibility Complex Class II, STAT3: Signal Transducer and Activator of Transcription 3, TGF: Transforming Growth Factor.)
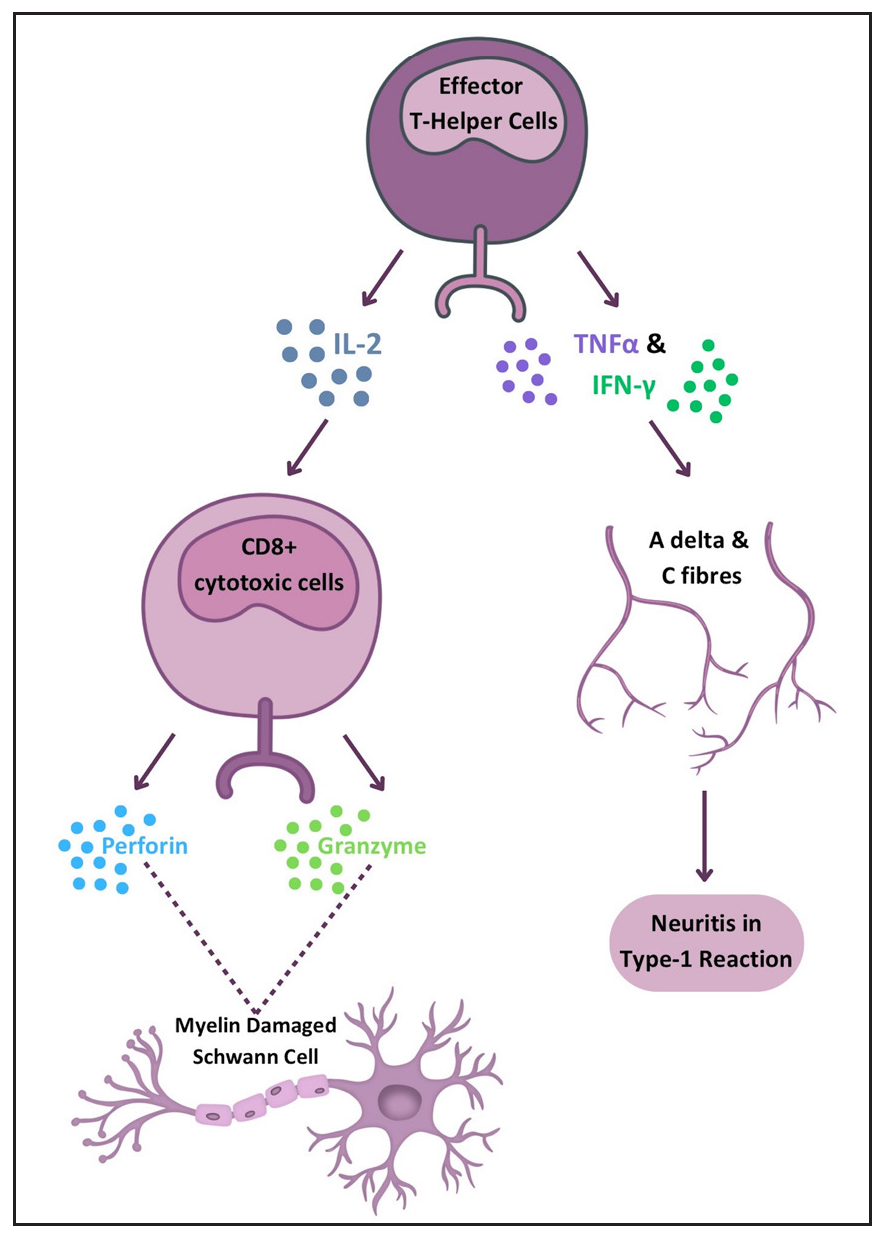
- Diagram illustrating the immune response in Type 1 reaction leading to neuritis and nerve damage. (CD8: Cluster of Differentiation 8, IFN: Interferon, IL: Interleukin, TNF: Tumor necrosis factor.)
Heat shock proteins (HSPs), expressed during chronic inflammation and stress, may induce autoimmune reactions during mycobacterial infections due to molecular mimicry with host proteins. Studies have identified B-cell mimicking epitopes shared between M. leprae’s HSP65 and host keratin, finding significantly higher antibody levels against specific HSP peptides in T1R patients compared to non-reaction individuals and healthy controls.6,7 These findings suggest potential predictive biomarkers for T1R and highlight the role of molecular mimicry in leprosy-induced immune responses. Unconventional T-cells are proposed to mediate the interface between innate and adaptive immunity in T1Rs. Pathak et al. revealed decreased γδ T-cell numbers and elevated NKT-like and NK cells in T1R patients compared to non-reaction individuals.8
IFN-γ is pivotal in T1Rs, with studies identifying increased IL-15 production and autophagy activation as downstream effects.9 Comparative gene expression analysis between T1R skin lesions and M. leprae + IFN-γ stimulated macrophages revealed 13 common genetic elements, including the autophagy regulator translocated promoter region (TPR), significantly increased in macrophages from T1R patients.9 Autophagy is crucial for inflammasome component degradation, limiting exaggerated inflammatory responses. Tuberculoid leprosy patients exhibit upregulated autophagy genes compared to lepromatous cases.10 Autophagy is upregulated by dead bacilli, while live mycobacteria inhibit it, serving as an immune escape mechanism.10 Downregulation of autophagy genes, along with TLR3 and NLRP3-IL-1β pathway overexpression in multibacillary (MB) patients, increases RR risk.11 Blocking autophagy with 3-methyladenine in M. leprae-stimulated monocytes enhances NLRP3 inflammasome expression and subsequent IL-1β and IL-6 production.11 Pro-autophagic drugs may help control bacillary load and potentially treat T1Rs.
Th17 population increases and T-regulatory cell (Treg) population reductions are observed in leprosy reactions, with decreased TGF-β and increased IL-6 and IL-21 production.12,13 IL-21 is crucial in T1R pathogenesis by promoting the differentiation of T-regulatory cells into the Th17 pathway, evidenced by its higher levels in T1R patients’ blood and skin lesions, positive correlation with Th17 markers, and negative correlation with Treg markers.14 Table 1 summarises novel mechanisms in T1R pathogenesis.
| Pathogenetic mechanism | Role in type 1 reaction | Evidence |
|---|---|---|
| Molecular mimicry | Monoclonal antibodies against M. leprae have been reported to cross-react with human nerve and skin components, suggesting a potential contribution to the development of autoimmune clinical manifestations in leprosy |
Molecular mimicry between cytokeratin-10 and HSP65 reported; elevated antibodies against HSP4 and HSP5 in type 1 reaction patients6,7 Differential antibody levels against mimicking peptides potentially predictive biomarkers for T1R development and leprosy disease |
| Unconventional T-cells | Mediating interface between innate and adaptive immunity |
Significant decrease in γδ T-cell numbers; elevated frequencies of NKT-like and NK cells in type 1 reactions8 Additionally, higher plasma levels of TNFα, IL1β, IL17 and CXCL10; increased gene expression of IFNγ, IP10, TNFα, IL6 and IL17A; upregulated chemokines like CCL3, CCR1, CCR5 and CXCR3 in Type 1 reaction patients8 |
| Autophagy |
Essential for inflammasome component degradation, limiting exaggerated inflammatory responses Downregulation of autophagy genes increases the risk of reversal reactions |
Comparative gene expression analysis revealed common genetic elements, including autophagy regulator TPR9 Upregulated autophagy genes in tuberculoid leprosy10 Live mycobacteria inhibit autophagy10 Downregulation of autophagy genes and TLR3, NLRP3-IL-1β pathway overexpression increase risk of reversal reactions11 Blocking autophagy enhances NLRP3 inflammasome expression11 |
| Th17 and Treg population alterations | Increase in Th17 and reduction in Treg populations observed |
IL-21, a differentiating cytokine promoting T-regulatory cells to Th17 pathway, explored14 IL-21+ cells significantly higher in T1R patients; gene expression of IL-21 correlates positively with Th17 cell markers and negatively with Treg cell markers14 |
HSP: Heat Shock Protein, TNFα: Tumour Necrosis Factor-alpha, IL1β: Interleukin 1-beta, IL17: Interleukin 17, CXCL10: C-X-C Motif Chemokine Ligand 10, IFNγ: Interferon-gamma, IP10: Interferon-gamma-Induced Protein 10, IL6: Interleukin 6, IL17A: Interleukin 17A, CCL3: C-C Motif Chemokine Ligand 3, CCR1: C-C Chemokine Receptor Type 1, CCR5: C-C Chemokine Receptor Type 5, CXCR3: C-X-C Chemokine Receptor Type 3, TPR: Translocated Promoter Region, TLR3: Toll-Like Receptor 3, NLRP3: Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3, Th17: T-helper 17 cell, Treg: Regulatory T-cell, NKT: Natural Killer T-cells.
Type 2 reactions
The pathogenesis of T2R involves type III hypersensitivity due to inadequate clearance of antigen-antibody complexes, resulting in inflammation and leukocyte chemotaxis. Skin biopsies of ENL patients show complement and immunoglobulin deposition in the dermis, similar to an Arthus reaction.15 Figure 3 illustrates the current knowledge regarding immunopathogenesis of T2R.
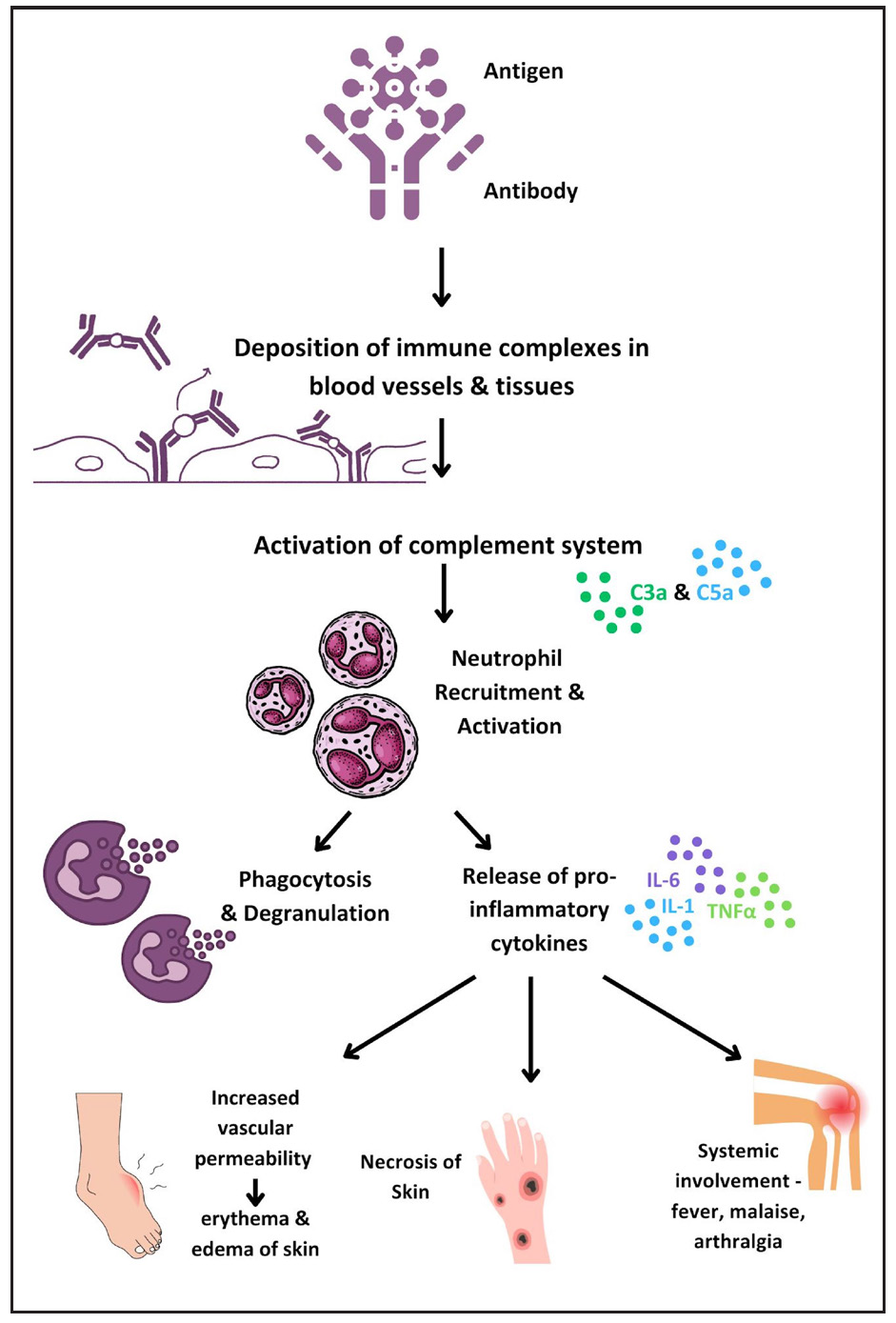
- Diagram illustrating the immunopathogenesis of type 2 reactions.
Neutrophils play a central role in ENL pathogenesis, with Tavares et al. highlighting elevated low-density neutrophils (LDNs) in ENL patients.16 Neutrophil extracellular traps (NETs), significant in severe autoimmune disorders, are abundant in ENL patients and reduced by thalidomide.17,18 The balance between IL-10 and TNF-α in neutrophils, along with Toll-like receptor 9 (TLR9) recognition of DNA, offers potential biomarkers for ENL.19,20 Further investigation revealed the upregulation of plasmacytoid dendritic cell/ type 1 interferon pathway, as a consequence to increase in TLR9 expression.21 B-cell subpopulation alterations22,23 and myeloid-derived suppressor cells with elevated annexin A1 levels may impact T-cell efficacy.24 Depleting CD25+ cells reversibly impairs immune response, highlighting Tregs as a potential therapeutic target.25 Neurotrophins, particularly nerve growth factor (NGF), showed decreased levels and the presence of autoantibodies modulated by cyclosporin A, suggesting targets for neuroimmune reaction control.26 Table 2 summarises recent breakthroughs in ENL and their therapeutic implications.
| Study | Salient findings | Implications |
|---|---|---|
| Tavares et al.16 |
Flow cytometry of ENL patients demonstrated elevated frequency of LDN, which displayed a neutrophilic-activated phenotype Higher CD11b and lower CD62L surface expressions on LDNs correlate with the activation status of LDNs ENL patients under thalidomide treatment presented similar frequency of LDNs as observed before treatment but its activation status was lower |
Potential biomarkers for diagnosis and monitoring of reactional states |
| Pacheco et al.19 |
Patients with ENL had a subpopulation of neutrophils that expressed IL-10R1 in both skin lesions and blood Neutrophils found in the blood of ENL patients were able to secrete detectable levels of TNF-α, which could be blocked by the addition of IL-10 |
IL-10R1 is a possible biomarker in ENL IL-10 pathway may be a therapeutic target for the management of ENL |
| Dias AA et al.20 | DNA sensing via TLR-9 constitutes a major innate immunity pathway involved in the pathogenesis and evolution of ENL | TLR-9 antagonists are potential alternative to more effectively treat ENL |
| Da Silva et al.18 |
Abundant NETs were found in T2R skin lesions and increased spontaneous NETs formation was observed in T2R peripheral neutrophils. TLR9 expression was shown to be higher in T2R neutrophils Treatment of T2R patients with thalidomide for seven consecutive days resulted in a decrease in all of the evaluated in vivo and ex vivo NETosis parameters. |
DNA recognition via TLR9 may be one of the pathways triggering this process during T2R, thus it is a potential therapeutic target |
| Nogueria et al.22 |
Compared to uninfected subjects, an increase in mature B-cells and a decrease in memory B-cells was observed in MB disease and ENL Decrease in atypical B-cells (CD27–CD21–) and an increase in activated B-cells (CD27+ CD21+) was noted during ENL episodes |
First study to describe the different B-cell phenotypes in polar forms of leprosy and in ENL |
| Negera et al.25 |
Increase in activated memory B-cells and reduced number of tissue-like memory B-cells in untreated ENL patients as compared to LL patient The percentage of total circulating B-cells was similar among ENL patients and non-reactional LL patients; however, proportion of B-cells was significantly reduced by treatment for ENL |
B-cell depletion may have a role in management of ENL |
| Da Silva et al.24 |
An increased density of myeloid-derived suppressor cells has been observed in patients with lepromatous leprosy and T2R Presence of annexin A1 was observed in all myeloid-derived suppressor cells. Monocytic myeloid-derived suppressor cell in the lepromatous patients had particularly higher levels of this protein when compared to the reactional patients |
High annexin A1 expression in lepromatous patients may be responsible for reduction in the efficacy of T-cell action against M. leprae, rendering the patient susceptible to MB disease |
| Jesus et al.26 |
NGF is decreased in the course of leprosy and there is presence of autoantibodies against NGF in all clinical forms of leprosy and neuroimmune reactions Levels of autoantibodies against NGF are decreased by the immunomodulatory activity of cyclosporin A, which mainly controls pain and improves motor function and sensitivity |
Suppression of anti-NGF and the regulation of NGF levels can be attractive targets for immunomodulatory treatment and for controlling the neuroimmune reactions of leprosy |
| Negera et al.25 |
Tregs in ENL maintain suppressive function despite reduced numbers Depletion of Tregs enhances TNFα and IFNγ responses, indicating a regulatory role |
Tregs emerge as potential targets for immune modulation in leprosy |
| Castro et al.13 |
Decrease in CD4+TGF-β+ Treg and CD8+ TGF-β+ Treg cells Upregulation of IL-17 and IL-6 |
Enhances our understanding of immune hyporesponsiveness in MB patients and hyperresponsiveness in reactions |
| Rosa et al.21 |
Increased type 1 IFN expression Decreased frequency of peripheral pDC |
pDC/type I IFN pathway may be utilised as potential biomarker for diagnosis Targeting pDC may be a viable therapeutic approach in ENL |
B-cells: B lymphocytes, CD11b: Cluster of Differentiation 11b, CD21: Cluster of Differentiation 21, CD27: Cluster of Differentiation 27, CD62L: Cluster of Differentiation 62L, ENL: Erythema Nodosum Leprosum, IFN: Interferon, IL-10: Interleukin-10, IL-10R1: Interleukin-10 Receptor Subunit Alpha, LDNs: Low-Density Neutrophils, MB: Multibacillary, NETs: Neutrophil Extracellular Traps, NGF: Nerve Growth Factor, pDC: Plasmacytoid dendritic cells, T2R: Type 2 Reaction, TLR-9: Toll-Like Receptor 9, TNF-α: Tumour Necrosis Factor Alpha
Infections are potential triggers for lepra reactions. Motta et al. found that 39% of patients with lepra reactions had concurrent infections, mainly oral.27 A thorough search based on patient history and clinical examination is advisable, especially in recurrent reactions. However, the impact of specific infections remains uncertain, as a scoping review did not indicate a higher incidence of reactions in patients with concurrent bacterial, fungal or parasitic infections.28
A recent study highlighted the different roles of dead and viable bacilli in the modulation of lepra reactions and nerve damage. While viable bacilli promote its own survival by exploiting the host cells, dead bacilli create a pro-inflammatory milieu and promote nerve damage.29
Diagnosis
Early diagnosis of reactions is essential to limit the resulting neural damage. Clinical examination remains the cornerstone of the diagnosis of a reaction once it manifests. Classically, T1R presents with erythema and oedema of pre-existing lesions which may or may not be associated with neuritis. ENL manifests as evanescent, tender nodules, more often associated with systemic complains like fever, joint pain, bone pain, and lymphadenopathy. Erythema Nodosum Leprosum International STudy (ENLIST) score is used frequently to classify it as mild, moderate or severe, which determines the management. There is an urgent need to identify biomarkers that may predict the course of the disease and identify patients who are at increased risk of developing reactional episodes. Currently, there is no diagnostic tool that reliably predicts the onset of reactions before clinical manifestations occur. Table 3 summarises the newer diagnostic modalities with a possible role in leprosy reactions.
| Type of diagnostic modality | Specific investigation | Results | Role in leprosy reactions |
|---|---|---|---|
| Non-invasive imaging | Dermoscopy35 |
T1R: intense erythema, large telangiectatic vessels, violaceous to brown periappendageal pigmentation, white globules, epidermal scaling, follicular plugging T2R: erythema, vascular dilation, red dots, hypopigmented structureless areas |
Diagnosis of leprosy reactions |
| HRUS with colour Doppler30 | Blood flow detected on colour Doppler in patients with nerve involvement in NCS and in those with minimal or no changes in NCS | Diagnosis of neural involvement in leprosy reactions with minimal nerve involvement or those lacking a motor response in NCS | |
| Magnetic resonance imaging31–33 |
Leprosy-associated peripheral nerve abscess usually reveals signal changes of an abscess with peripheral contrast enhancement of the abscess wall and inflammatory thickening of the whole nerve trunk Rarely, CNS lesions may also be diagnosed by MRI |
Differentiating many mass lesions of peripheral nerves such as a schwannoma, neurofibroma and more from leprosy-associated abscess or cranial nerve involvement | |
| Serology for M. leprae | Anti-PGL-1 antibody37 | Moderate sensitivity and specificity for predicting future reactions | May predict risk of future ENL episodes, but not type 1 reaction |
| Anti-NDO-LID-1 antibody37 | Good sensitivity and specificity for predicting future reactions | ||
| Anti LAM salivary antibody39 | Similar odds of predicting reaction as anti-PGL-1 antibody | ||
| Inflammatory markers | NLR, PLR and LMR41 | Fair sensitivity and specificity in diagnosis of leprosy reactions | Diagnosis and possibly response to anti-inflammatory therapies |
| C1q42 | Lower level during ENL, with normalization after treatment | Diagnosis of leprosy reactions and monitoring response to treatment | |
| Cyclooxygenase 2 and vascular endothelial growth factor expression in dermal macrophages and vascular endothelium43 | Higher in type 1 followed by T2R compared to controls | Potential targets for treatment of lepra reactions | |
| Pentraxin-344 | Elevated prior to onset and during acute ENL, declined after treatment | Prediction of predisposition to ENL, diagnosis of ENL and monitoring response to therapy | |
| Molecular diagnosis | Dual colour reverse-transcription multiplex ligation-dependent probe amplification45 | A transcriptomic signature of risk for reversal reactions consisting of five genes (CCL2, CD8A, IL2, IL15andMARCO) was identified based on cross-sectional comparison of RNAexpression | Could predict reversal reactions at least two weeks before onset |
Anti-LAM salivary antibody: Antibodies against lipoarabinomannan, Anti-NDO-LID-1 antibody: Anti-natural octyl disaccharide-leprosy IDRI diagnostic, Anti-PGL-1 antibody: Antibodies against Phenolic Glycolipid 1, C1q: Complement 1q, CNS: Central nervous system, ENL: Erythema nodosum leprosum, HRUS: High resolution ultrasound, LMR: Lymphocyte-to-Monocyte Ratio, MRI: Magnetic resonance imaging, NCSs: Nerve Conduction Studies, NLR: Neutrophil-to-Lymphocyte Ratio, Pentraxin-3: Pentraxin-related protein 3, PLR: Platelet-to-Lymphocyte Ratio, T1R: Type 1 Reaction, T2R: Type 2 Reaction
Nerve conduction study (NCS) is established for detecting neural dysfunction. Recent studies suggest high-resolution ultrasonography (HRUS) with colour Doppler (CD) can aid in diagnosing leprosy reactions, especially in patients with minimal nerve involvement or lacking a motor response in NCS. HRUS with CD is particularly useful for detecting reactions in nerves with minor changes or no motor response in NCS.30 Magnetic resonance imaging (MRI) helps diagnose nerve abscesses, distinguish leprosy reactions from other causes of nerve thickening and detect the central nervous system involvement.31–33 Dermoscopy is another easily available non-invasive imaging which has been utilised in leprosy reactions.34,35 Table 3 outlines the dermoscopic features of lepra reactions.
Higher titres of antibodies against natural octyl disaccharide-leprosy IDRI diagnostic (anti-NDO-LID) at baseline have been associated with the risk of future ENL.36 An observational study showed that patients with ENL had 66.66% higher titres of antibodies against Phenolic Glycolipid-1 (anti-PGL-1) and 91.66% higher titres of anti-NOD-LID-1 as compared to those without reactions.37 Further, positivity for both antibodies was significantly associated with the likelihood of developing reactions in the future. Another study revealed anti-PGL1 to be an important prognostic factor for the prediction of leprosy reactions.38 Additionally, salivary antibodies to lipoarabinomannan (LAM) antigen may be used as a tool to monitor patients undergoing treatment to predict reactional episodes.39
Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) have been utilised to assess the inflammatory response in various diseases. Gomes et al. assessed the utility of assessing NLR in patients with leprosy reactions.40 The NLR cut-off for the diagnosis of any leprosy reaction was 2.75 (sensitivity 61.0%, specificity 92.0%, accuracy 77.0%) while the cut-off for T2R was 2.95 (sensitivity 81.0%, specificity 74.0%, accuracy 78.0%). Another study noted that NLR and PLR but not LMR were useful as a diagnostic biomarker for ENL.41
Complement component 1q (C1q), a key component of the classical complement pathway, has been studied as a diagnostic and monitoring parameter for ENL.42 C1q levels in peripheral blood were significantly lower in untreated ENL patients compared to LL controls. Additionally, increased genetic expression of C1q components was noted in ENL patients’ peripheral blood and skin biopsies, which normalised after treatment, suggesting C1q as a potential diagnostic marker and treatment response indicator for ENL. An immunohistochemical study also found significantly increased expression of cyclooxygenase 2 and vascular endothelial growth factor in dermal macrophages and vascular endothelium in T1Rs, followed by T2Rs, compared to controls.43
Pentraxin-3 (PTX-3), an inflammatory marker, is higher in MB patients before and during acute ENL and decreases within seven days of thalidomide therapy.44 A novel study used RNA expression analysis of 1090 whole blood samples to profile 103 target genes for innate and adaptive immune responses.45 A transcriptomic signature of five genes (CCL2, CD8A, IL2, IL15, MARCO) was identified, predicting RRs at least two weeks before onset.45 Other potential ENL diagnostic biomarkers include IL-6, IL-7, CCL-11, alpha-1 acid glycoprotein and CD-64.46 No single marker or a set of markers has shown to reliably and effectively prognosticate the occurrence of lepra reactions.
The Specialist System for Evaluation of Risk of Occurrence of Reactional States in Leprosy (SEPAREH) is an online tool that predicts leprosy reactions with up to 87.7% accuracy using socio-demographic details, family history, clinical, laboratory and genetic data.47
Treatment
Type 1 reactions
The objective of treatment for T1R is control of neural inflammation and prevention of further immune-mediated damage. For mild T1R, non-steroidal anti-inflammatory drugs may suffice, but severe reaction, especially with neuritis, requires medical and/or surgical treatment. Current evidence suggests that oral corticosteroids are the mainstay for severe T1Rs and should be started in a dose of 0.5–1 mg/kg/day and gradually tapered to zero over a span of 20 weeks [Figure 4]. A randomised trial (Treatment of Early Neuropathy in Leprosy or TENLEP) compared oral corticosteroids for 20 and 32 weeks in patients with recent onset nerve function impairment (less than six months duration), including T1R.48 No difference in clinical outcomes was noted between the groups at the end of the study period.
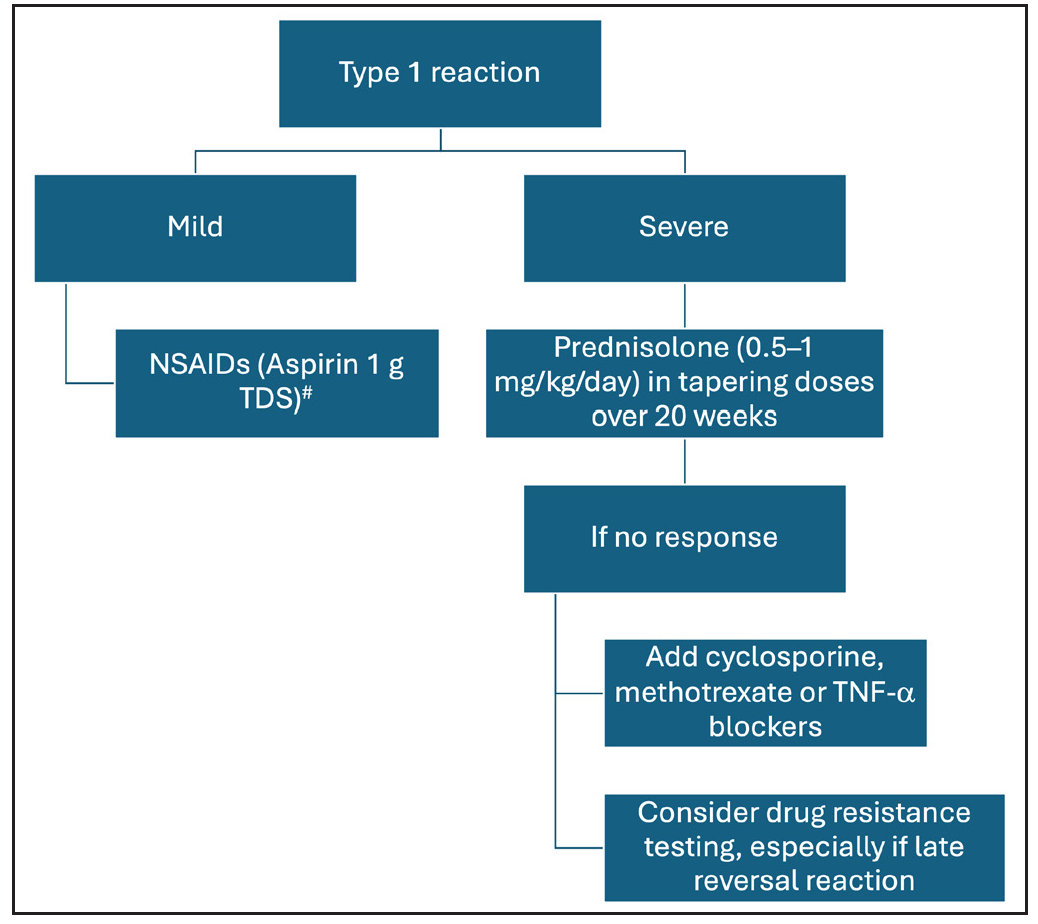
- Management of type 1 leprosy reactions (NSAIDs: non-steroidal anti-inflammatory drugs, TDS: Ter Die Sumendum or thrice a day, TNF-α: tumour necrosis factor alpha). #Consider combination with proton pump inhibitors to mitigate associated gastrointestinal adverse effects.
Other immunosuppressive agents that have been used successfully for the management of T1R include cyclosporine and methotrexate.49-51A recent review and systematic analysis has highlighted the role of methotrexate in type 1 and 2 lepra reactions. The study recommends lower doses of methotrexate than the dose used in autoimmune diseases and its combination with low-dose steroids.52 A combination of azathioprine with prednisolone was not found to be superior over prednisolone alone.53 Further, incidence of anaemia was increased by a combination of azathioprine and dapsone.
Successful use of infliximab as a therapy for RR was reported recently in a patient with steroid-dependent neuritis.54 A dramatic and lasting remission was noted after three infliximab infusions at week 0, 1 and 6. However, it must be noted that infliximab has been implicated in causing neuritis and demyelination, and should be used with utmost caution in a patient with nerve function impairment. In cases of recalcitrant neuritis or nerve abscess, surgical decompression may be warranted, based on the physician’s discretion.55
Type II reactions
Figure 5 summarises the management of T2R. Rest and non-steroidal anti-inflammatory agents are the cornerstone therapy for mild ENL. Use of corticosteroids is recommended by WHO guidelines for severe ENL56; however, it is associated with several adverse effects, which may be minimised by splitting the dose of oral corticosteroids.57 Thalidomide is an effective and rapidly acting therapy for severe ENL that acts by inhibition of TNF-α. Its use is restricted by potential teratogenicity, neuropathy and limited availability. A recent real-world study demonstrated the low-dose thalidomide (25–150 mg/day) to be as efficacious as the high-dose regimen.58 Clofazimine is another important agent for the management of chronic ENL; however, the slow onset of action limits its use in cases of severe/acute ENL. Two pilot studies from Ethiopia analysed the role of cyclosporine in the management of ENL.49 Cyclosporine demonstrated potential benefits in acute ENL, reducing severity and prednisolone requirement, but showed less efficacy in chronic ENL, leading to earlier and more severe flare-ups requiring higher prednisolone doses. There is an unmet need for safe and effective therapies for the management of ENL.
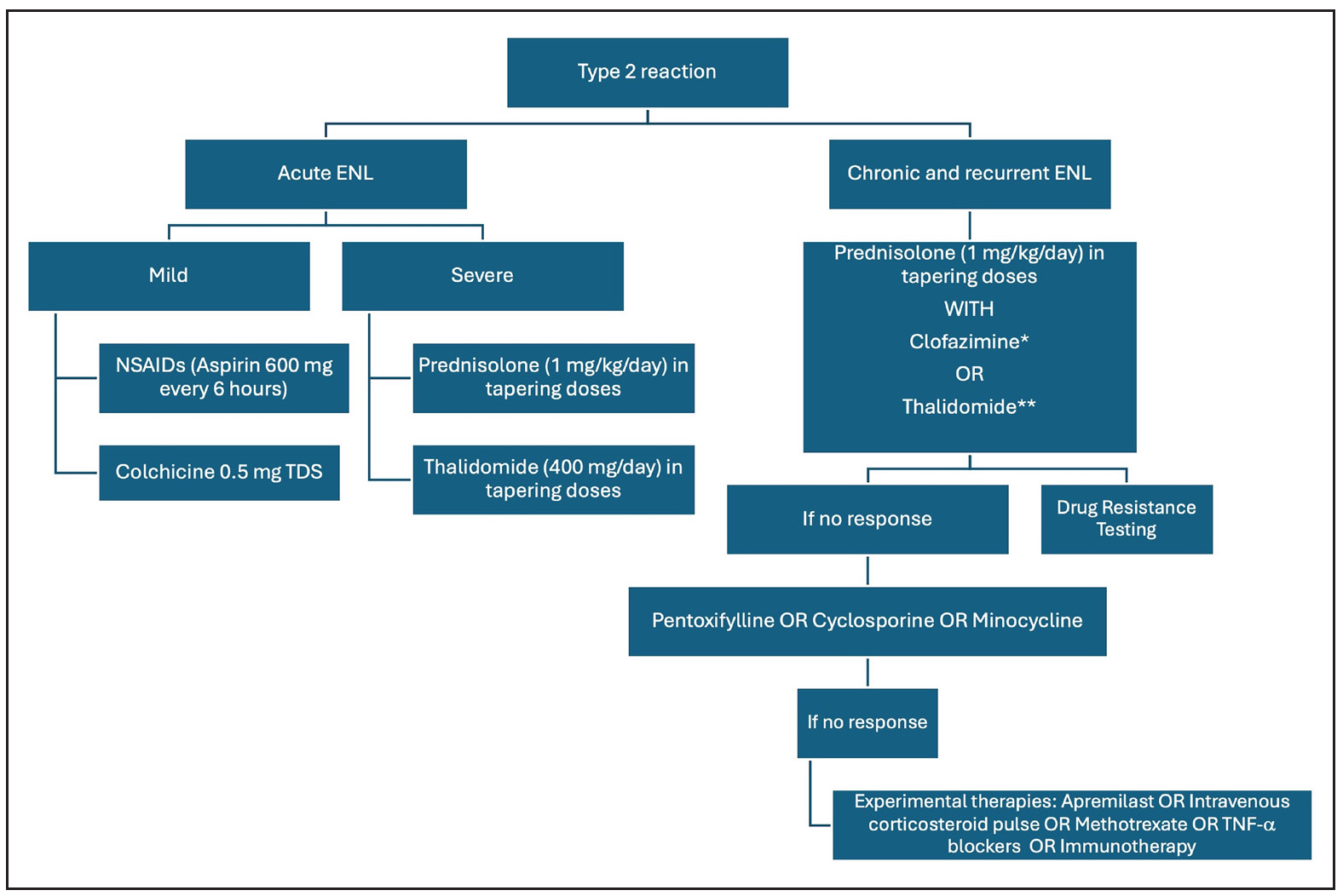
- Management of type 2 leprosy reactions (ENL: erythema nodosum leprosum, NSAIDs: non-steroidal anti-inflammatory drugs; TNF-α: tumour necrosis factor alpha). *Clofazimine in a dose of 100 mg TDS for 12 weeks, 100 mg BD for 12 weeks and 100 mg OD for 12–24 weeks. **Thalidomide up to a dose of 400 mg/day in tapering doses, #Consider combination with proton pump inhibitors to mitigate associated gastrointestinal adverse effects
Several therapies show promise in ENL, including methotrexate, apremilast, minocycline, colchicine and TNF-α inhibitors. A review of 21 patients treated with methotrexate (7.5–20 mg) found it safe and effective for leprosy reactions when combined with low-dose corticosteroids.59 Its role in ENL was highlighted in a recent trial, though the lack of controlled studies makes standard dosage protocols difficult.52 It is particularly useful for patients with steroid contraindications like diabetes. An ongoing trial (MaPs in ENL) is assessing methotrexate’s efficacy in ENL.60 However, careful patient selection is needed due to potential overlap with dapsone induced side effects and methotrexate side effects like anaemia and liver issues.61
In a case series of six ENL patients, etanercept combined with corticosteroids reduced steroid dosage by 42%.62 A systematic review found four reports of ENL successfully treated with infliximab or etanercept,63 but also noted ten cases of leprosy following TNF-α inhibitor use, suggesting a risk of leprosy infection or reactivation of subclinical infection.63
Apremilast, a phosphodiesterase-4 inhibitor, reduces pro-inflammatory cytokines, like TNF-α and IL-12/23, involved in ENL pathogenesis. Case reports and series have shown its successful use in chronic and recurrent ENL.64–66 Minocycline, an antibacterial and anti-inflammatory agent, is used as a second-line anti-leprosy treatment. A pilot study found minocycline effective in eight out of ten recalcitrant ENL patients.67 A randomised controlled trial comparing clofazimine and minocycline in chronic and recurrent ENL showed that minocycline provided earlier control, longer remission and fewer side effects, though both groups had similar flare-ups and additional prednisolone requirements.68 Colchicine has also been repurposed for ENL.69 A network meta-analysis indicated potential efficacy for clofazimine, followed by dapsone+ rifampicin, in treating type 2 leprosy reactions.70 However, in practice, immunosuppressants or immunomodulatory agents are often needed for managing severe reactions.
MDT is often restarted to control chronic/recurrent ENL. An alternative regimen with minocycline, clofazimine and ofloxacin may be effective for patients with high bacillary loads and frequent reactions.71 However, the role of MDT in managing reactions is contentious, with inconsistent efficacy reported.72 Immunotherapy, especially Mycobacterium indicus pranii (MIP), has shown promise in preventing and controlling ENL episodes.73–75 Gupta et al. reported a patient with ENL refractory to multiple treatments who responded well to a single dose of MIP vaccine, though rare exacerbations have been noted.76,77 High-quality data is limited, making conclusive recommendations difficult. An individualised approach, such as MIP or second-line therapies, may benefit those with high bacillary loads and poor responses to MDT. Apremilast, minocycline and colchicine are useful for ENL patients with co-infections or corticosteroid dependence. IL-17 and IL-6 blockers, metformin and SSRIs are potential therapies awaiting clinical studies. Table 4 summarises the current evidence on newer ENL therapies.
| Medication name | Indication (no. of patients) | Mechanism of action in ENL | Dosage schedule | Efficacy | Adverse effects |
|---|---|---|---|---|---|
| Methotrexate59 | Recurrent ENL (n=15) | Increased adenosine production, reduction of pro-inflammatory cytokines and increase in anti-inflammatory cytokines | 7.5–20 mg weekly (median 15 mg) in combination with corticosteroids | Improvement in reactional symptoms in all reported cases | No serious adverse effects reported in any of the case reports |
| Cyclosporine49 | Acute ENL (n=13) | Inhibits IL-2 production by inhibiting calcineurin, decreased T-cell proliferation | 7.5 mg/kg/day in combination with prednisolone, tapered to 2 mg/kg/day in 16 weeks | 1.29 recurrences per patient in 32 weeks, mean time to first recurrence was 23 weeks | Hypertrichosis, gum hyperplasia, hypertension |
| Chronic ENL (n=20) | 2.3 recurrences per patient in 32 weeks, mean time to first recurrence was 7.1 weeks | ||||
| Minocycline68 | Chronic and recurrent ENL (n=30) | Anti-inflammatory, inhibition of neutrophil chemotaxis and downregulation of pro-inflammatory cytokines | 100 mg once a day for three months | Initial control of reaction in 2.97 ± 1.9 weeks, flares in 55.2% patients during follow-up of nine months | Blue gray pigmentation, pain abdomen/epigastric pain |
| Apremilast65 | Chronic or recurrent ENL (n=12) | Structure homology with thalidomide, PDE-4 inhibition, depletion of pro-inflammatory cytokines | 30 mg twice daily for six months (after initial standard dose titration) in combination with oral corticosteroids | Resolution of symptoms and signs in 15.63 ± 6.36 days, recurrence of ENL after stoppage of steroids in 27.2% patients | Gastrointestinal adverse effects, urticaria |
| TNF-α inhibitors (infliximab and etanercept)63 | Refractory ENL (n=5) | Disruption of pro-inflammatory cascade by blockade of TNF-α |
Infliximab: 5 mg/kg IV at day zero, repeated at two and six weeks Etanercept: 50 mg/week subcutaneously (case reports of use for up to 12 months) |
Rapid improvement in all patients as early as 48 hours | None reported |
ENL: erythema nodosum leprosum, IL: interleukin, PDE-4: phosphodiesterase-4, TNF- α: tumour necrosis factor-alpha
Another recent aspect in management of patients with chronic recalcitrant ENL, especially in cases of diffuse LL, is the presence of M. lepromatosis infection. These patients most often present with necrotic lesions which resemble the Lucio phenomenon. Hemi-nested PCR targeting 16S rRNA gene specific for M. lepromatosiscan be utilised for diagnosis. Further studies are needed to elucidate the therapeutic implications of the diagnosis of M lepromatosis.78
Recent studies highlight the role of resistance in type 1 and 2 lepra reactions and the need for modulation of the treatment accordingly.72 Narang et al. suggest testing for resistance in all cases of refractory chronic/recurrent ENL.79
Prevention
Reactions result from abrupt immune fluctuations, making immunotherapy crucial for managing the immune milieu. Most studies support immunotherapy’s preventive role in reactions. Bacillus Calmette–Guérin (BCG) vaccine was first reported to be efficacious in leprosy in 1939, where lepromin conversion was noted in 90% of healthy children after receiving BCG. The incidence of reactions and hence the disability and neuritis were found to be significantly lower in vaccinated patients.80 A study comparing the effect of addition of BCG or MIP compared to placebo showed least incidence of T2R in the BCG arm.75 However, Shetty et al. found no notable response in bacterial or granuloma clearance after administering BCG post-MDT.81 Despite higher reaction rates with BCG, nerve function impairment was lower.
Mw, now renamed as MIP, is a rapidly growing, non-pathogenic mycobacterium developed by Talwar et al. Immunisation with MIP every three months in combination with MDT led to faster clinical improvement, faster bacillary clearance and shortened duration of MDT.82,83 A double-blind trial showed higher early phase reactions with MIP due to its immunomodulatory action, but reduced reactions overall after six months, lowering morbidity.84 Paradoxical induction of four ENL episodes recurring with each three-monthly dose of MIP vaccine was recently reported in a patient with histoid leprosy.77
Incorporating MIP vaccination into India’s national program is cost-effective, with an incremental cost-effectiveness ratio (ICER) of ₹73,790 per Quality-Adjusted Life Year (QALY) gained by vaccinating both new cases and their contacts over five years.85 A trial comparing MIP and BCG efficacy showed one type 2 and one mild T1R among 14 MIP patients, while five out of ten BCG patients had ENL, with four requiring corticosteroids.86
LepVax, a hybrid recombinant vaccine with cross-linked antigens in a Glucopyranosyl Lipid Adjuvant in Stable Emulsion (GLA-SE) emulsion, reduced sensory nerve damage and delayed motor nerve damage in armadillos.87 Unlike BCG, LepVax did not induce nerve injury in experimental leprosy models. It has shown safety and immunogenicity in a phase 1 trial in healthy adults, but its role as immunotherapy needs further exploration.88
Challenges and future directions
The management of leprosy reactions presents significant challenges, particularly with the rising incidence of chronic or recurrent reactions and the emergence of recalcitrant cases. There is a pressing need for biomarkers capable of predicting the disease course and guiding treatment decisions, addressing the imperative for personalised therapeutic approaches in reaction management. Further, the integration of prevention strategies in management of leprosy cases, especially the ones with reactional episodes, cannot be emphasised enough.
Conclusion
Leprosy continues to be an enigma, with several gaps in our knowledge regarding the pathogenesis of leprous reactions. Lack of representative animal models and sudden onset of reactions make it difficult to evaluate the disease pathophysiology. Reliable biomarkers that may be measured longitudinally to monitor treatment response are being explored. Several therapies are currently undergoing trials for leprosy reactions. As the pathogenesis of disease continues to be unravelled, targeted therapies may be the future for safe and effective management of this debilitating and stigmatising infection.
Acknowledgements
We express our gratitude to Ms. Khushi Jain and Dr. Ishita Kaushal for their valuable assistance with the graphical content.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Leprosy type 1 (reversal) reactions and their management. Lepr Rev. 2008;79:372-86.
- [CrossRef] [PubMed] [Google Scholar]
- A Systematic Review of Immunological Studies of Erythema Nodosum Leprosum. Front Immunol. 2017;8:233.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Leprosy: Clinical and immunopathological characteristics. An Bras Dermatol. 2022;97:338-47.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Leprosy: Treatment and management of complications. J Am Acad Dermatol. 2020;83:17-30.
- [CrossRef] [PubMed] [Google Scholar]
- Type 1 reaction in leprosy: A model for a better understanding of tissue immunity under an immunopathological condition. Expert Rev Clin Immunol. 2015;11:391-407.
- [CrossRef] [PubMed] [Google Scholar]
- Mimicking B and T cell epitopes between Mycobacterium leprae and host as predictive biomarkers in type 1 reaction in leprosy. Sci Rep. 2021;11:24431.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular mimicry between HSP 65 of mycobacterium leprae and cytokeratin 10 of the host keratin; role in pathogenesis of leprosy. Cell Immunol. 2012;278:63-75.
- [CrossRef] [PubMed] [Google Scholar]
- Unveiling the role of NK cells, NKT-like cells, and γδ cells in pathogenesis of type 1 reactions in leprosy. Heliyon. 2024;10:e25254.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autophagy-associated IL-15 production is involved in the pathogenesis of leprosy type 1 reaction. Cells. 2021;10:2215.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autophagy Is an innate mechanism associated with leprosy polarization. PLoS Pathog. 2017;13:e1006103.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autophagy impairment is associated with increased inflammasome activation and reversal reaction development in multibacillary leprosy. Front Immunol. 2018;9:1223.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Leprosy reactions show increased Th17 cell activity and reduced FOXP3+ tregs with concomitant decrease in TGF-β and increase in IL-6. PLoS Negl Trop Dis. 2016;10:e0004592.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Downmodulation of regulatory T cells producing TGF-β participates in pathogenesis of leprosy reactions. Front Med (Lausanne). 2022;9:865330.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IL-21 plays an important role in modulating “Th17-Treg” cell axis in leprosy Type 1 reactions. Cytokine. 2022;152:155821.
- [CrossRef] [PubMed] [Google Scholar]
- A Systematic Review of Immunological Studies of Erythema Nodosum Leprosum. Front Immunol.. 2017;8:233.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mycobacterium leprae induces neutrophilic degranulation and low-density neutrophil generation during erythema nodosum leprosum. Front Med (Lausanne). 2021;8:711623.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neutrophil NET working in ENL: Potential as a putative biomarker: Future Insights. Front Med (Lausanne). 2021;8:697804.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neutrophil extracellular traps contribute to the pathogenesis of leprosy type 2 reactions. PLoS Negl Trop Dis. 2019;13:e0007368.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Erythema nodosum leprosum neutrophil subset expressing il-10r1 transmigrates into skin lesions and responds to IL-10. Immunohorizons. 2020;4:47-56.
- [CrossRef] [PubMed] [Google Scholar]
- DNA Sensing via TLR-9 constitutes a major innate immunity pathway activated during erythema nodosum leprosum. J Immunol. 2016;197:1905-13.
- [CrossRef] [PubMed] [Google Scholar]
- The Type I interferon pathway is upregulated in the cutaneous lesions and blood of multibacillary leprosy patients with erythema nodosum leprosum. Front Med (Lausanne). 2022;9:899998.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Changes in B cell pool of patients with multibacillary leprosy: Diminished memory B cell and enhanced mature B in peripheral blood. Front Immunol. 2021;12:727580.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Increased activated memory B-cells in the peripheral blood of patients with erythema nodosum leprosum reactions. PLoS Negl Trop Dis. 2017;11:e0006121.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Analysis of the myeloid-derived suppressor cells and annexin A1 in multibacillary leprosy and reactional episodes. BMC Infect Dis. 2021;21:1050.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Regulatory T cells in erythema nodosum leprosum maintain anti-inflammatory function. PLoS Negl Trop Dis. 2022;16:e0010641.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cyclosporin A as an alternative neuroimmune strategy to control neurites and recover neuronal tissues in leprosy. Neuroimmunomodulation. 2022;29:15-20.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy reactions: Coinfections as a possible risk factor. Clinics (Sao Paulo). 2012;67:1145-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bacterial, fungal and parasitic co-infections in leprosy: A scoping review. PLoS Negl Trop Dis. 2023;17:e0011334.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gene expression profile of mycobacterium leprae contribution in the pathology of leprosy neuropathy. Front Med (Lausanne). 2022;9:861586.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison between nerve conduction study and high-resolution ultrasonography with color doppler in type 1 and type 2 leprosy reactions. Clin Neurophysiol Pract. 2021;6:97-102.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multiple nerve abscesses: An unusual manifestation of lepra reaction in a patient with borderline leprosy. Neurol India. 2016;64:1326-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-cranial involvement of trigeminal nerve in a patient with borderline tuberculoid leprosy in type 1 lepra reaction. Australas J Dermatol. 2023;64:e87-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging in leprosy: the nerves and beyond. Radio Inf Dis. 2020;7:12-21. Available from: http://dx.doi.org/10.1016/j.jrid.2020.03.008
- [CrossRef] [Google Scholar]
- Dermatoscopy in leprosy and its correlation with clinical spectrum and histopathology: A prospective observational study. J Eur Acad Dermatol Venereol. 2019;33:1947-51.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in leprosy: A clinical and histopathological correlation study. Dermatol Pract Concept. 2021;11:e2021032.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Utility and limitations of serodiagnostic tests in monitoring the response to treatment of leprosy patients. Diagn Microbiol Infect Dis. 2020;96:114984.
- [CrossRef] [PubMed] [Google Scholar]
- Can anti-PGL-1 and anti-NDO-LID-1 antibody titers be used to predict the risk of reactions in leprosy patients? Diagn Microbiol Infect Dis. 2018;91:260-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, epidemiological, and laboratory prognostic factors in patients with leprosy reactions: A 10-year retrospective cohort study. Front Med (Lausanne). 2022;9:841030.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-lipoarabinomannan-specific salivary IgA as prognostic marker for leprosy reactions in patients and cellular immunity in contacts. Front Immunol. 2018;9:1205.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnostic value of neutrophil-to-lymphocyte ratio in patients with leprosy reactions. Heliyon. 2020;6:e03369.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnostic value of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio in the diagnosis of erythema nodosum leprosum: A retrospective study. Trop Med Infect Dis. 2022;7:39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Complement C1q expression in erythema nodosum leprosum. PLoSNegl Trop Dis. 2018;12:e0006321.
- [CrossRef] [Google Scholar]
- Cyclooxygenase 2 and vascular endothelial growth factor-potential targets to manage lepra reactions: A case-control study. Dermatol Ther. 2021;34:e14882.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated pentraxin-3 concentrations in patients with leprosy: potential biomarker of erythema nodosum leprosum. J Infect Dis. 2017;216:1635-43.
- [CrossRef] [PubMed] [Google Scholar]
- Whole blood RNA signatures in leprosy patients identify reversal reactions before clinical onset: A prospective, multicenter study. Sci Rep. 2019;9:17931.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- What is new in the pathogenesis and management of erythema nodosum leprosum. Indian Dermatol Online J. 2020;11:482-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prediction of the occurrence of leprosy reactions based on Bayesian networks. Front Med (Lausanne). 2023;10:1233220.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effectiveness of 32 versus 20 weeks of prednisolone in leprosy patients with recent nerve function impairment: A randomized controlled trial. PLoSNegl Trop Dis. 2017;11:e0005952.
- [CrossRef] [Google Scholar]
- Comparison of efficacy and safety of ciclosporin to prednisolone in the treatment of erythema nodosum leprosum: Two randomised, double blind, controlled pilot studies in Ethiopia. PLoSNegl Trop Dis. 2016;10:e0004149.
- [CrossRef] [Google Scholar]
- Response to ciclosporin treatment in Ethiopian and Nepali patients with severe leprosy Type 1 reactions. Trans R Soc Trop Med Hyg. 2007;101:1004-12.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate treatment for type 1 (reversal) leprosy reactions. Clin Infect Dis. 2007;45:e7-9.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate as a corticosteroid-sparing agent in leprosy reactions: A French multicenter retrospective study. PLoSNegl Trop Dis. 2023;17:e0011238.
- [Google Scholar]
- AZALEP a randomized controlled trial of azathioprine to treat leprosy nerve damage and type 1 reactions in India: Main findings. PLoSNegl Trop Dis. 2017;11:e0005348.
- [CrossRef] [Google Scholar]
- Infliximab in leprosy type 1 reaction: A case report. Int J Dermatol. 2021;60:1285-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of peripheral neuropathy in leprosy: The case for nerve decompression. Plast Reconstr Surg Glob Open. 2016;4:e637.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Leprosy/Hansen Disease: Management of reactions and prevention of disabilities [Internet]. Available from: https://www.who.int/publications/i/item/9789290227595
- A rarely employed therapeutic pearl of split-dose oral corticosteroid in severe type 2 or erythema nodosum leprosum reaction in lepromatous leprosy and its therapeutic rationale. Indian J Dermatol. 2022;67:425-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A real-world study of low-dose thalidomide in severe erythema nodosum leprosum highlighting its mechanistic rationale in a resource-constrained target population. Int J Dermatol. 2023;62:48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Experience of a referral center and systematic review of the literature. Travel Med Infect Dis. 2020;37:101670.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate and prednisolone study in erythema nodosum leprosum (MaPs in ENL) protocol: A double-blind randomised clinical trial. BMJ Open. 2020;10:e037700.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methotrexate in erythema nodosum leprosum: Pitfalls to avoid. Trop Doct. 2022;52:226-7.
- [CrossRef] [PubMed] [Google Scholar]
- Can etanercept be an option in management of recurrent steroid-dependent erythema nodosum leprosum? A retrospective study of six patients. Indian J Dermatol Venereol Leprol. 2022;88:243-6.
- [CrossRef] [PubMed] [Google Scholar]
- Biologics in leprosy: A systematic review and case report. Am J Trop Med Hyg. 2020;102:1131-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Successful treatment of erythema nodosum leprosum with apremilast. Dermatol Ther. 2022;35:e15258.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast in multibacillary leprosy patients with chronic and recurrent erythema nodosum leprosum: A prospective single-centre pilot study. J EurAcad Dermatol Venereol. 2021;35:e917-9.
- [Google Scholar]
- Apremilast in chronic recalcitrant erythema nodosum leprosum: A report of two cases. Br J Dermatol. 2020;182:1034-7.
- [CrossRef] [PubMed] [Google Scholar]
- Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the efficacy and safety of minocycline and clofazimine in chronic and recurrent erythema nodosum leprosum – A randomized clinical trial. Dermatol Ther. 2021;34:e15125.
- [CrossRef] [PubMed] [Google Scholar]
- A case of recurrent steroid-dependent severe type 2 lepra reaction treated successfully with colchicine. Int J Mycobacteriol. 2021;10:472-4.
- [CrossRef] [PubMed] [Google Scholar]
- Seventy years of evidence on the efficacy and safety of drugs for treating leprosy: A network meta-analysis. J Infect. 2023;86:338-51.
- [CrossRef] [PubMed] [Google Scholar]
- Alternate anti-leprosy regimen for multidrug therapy refractory leprosy: A Retrospective study from a tertiary care center in North India. Am J Trop Med Hyg. 2019;100:24-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective case control study of resistance to rifampicin, dapsone and ofloxacin in Type 1 and Type 2 leprosy reactions and the therapeutic impact of modified treatment regimen on reactions. J Eur Acad Dermatol Venereol. 2023;37:e149-51.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of combined Mycobacterium w vaccine and 1 year of MDT on multibacillary leprosy patients. Int J Lepr Other Mycobact Dis. 2001;69:187-94.
- [PubMed] [Google Scholar]
- 10–12 years follow-up of highly bacillated BL/LL leprosy patients on combined chemotherapy and immunotherapy. Vaccine. 2004;22:3649-57.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2005;73:105-14.
- [PubMed] [Google Scholar]
- Chronic recalcitrant erythema nodosum leprosum: Therapeutic dilemma and role of mycobacterium indicus pranii vaccine. An Bras Dermatol. 2022;97:49-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent erythema nodosum leprosum associated with Mycobacterium indicus pranii vaccine in a case of leprosy: A rare paradox. J Eur Acad Dermatol Venereol. 2021;35:e391-3.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic characterization of Mycobacterium lepromatosis from ENL patients from India. Infect Genet Evo l. 2023;116:105537.
- [CrossRef] [Google Scholar]
- Equal rates of drug resistance in leprosy cases with relapse and recurrent/chronic Type 2 reaction: Time to revise the guidelines for drug-resistance testing in leprosy? Clin Exp Dermatol. 2022;47:297-302.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of BCG Vaccination on evolution of leprosy. Med J Armed Forces India. 2005;61:26-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- BCG immunotherapy as an adjunct to chemotherapy in BL-lL patients—its effect on clinical regression, reaction severity, nerve function, lepromin conversion, bacterial/antigen clearance and ‘persister’ M. leprae. Lepr Rev. 2013;84:23-40.
- [PubMed] [Google Scholar]
- Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, mycobacterium w in multibacillary leprosy patients. Vaccine. 1990;8:121-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium w vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy: A report on hospital based immunotherapeutic clinical trials with a follow-up of 1–7 years after treatment. Lepr Rev. 2000;71:179-92.
- [CrossRef] [PubMed] [Google Scholar]
- Addition of Mycobacterium indicus pranii vaccine as an immunotherapeutic to standard chemotherapy in borderline leprosy: A double-blind study to assess clinical improvement (preliminary report) Br J Dermatol. 2017;176:1388-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cost-effectiveness of incorporating Mycobacterium indicus pranii vaccine to multidrug therapy in newly diagnosed leprosy cases for better treatment outcomes &immunoprophylaxis in contacts as leprosy control measures for National Leprosy Eradication Programme in India. Indian J Med Res. 2021;154:121-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective study to evaluate the bacteriological and antigen-specific immunological responses induced by MIP and/ BCG vaccines as adjunctive treatment (immunotherapy) in multibacillary leprosy patients treated with multidrug therapy 2020 Oct 20 Identifier CTRI/2020/11/028803, 2020 Oct 20; [1 page]. https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=NDgyNzU=&Enc=&userName=mip%20vaccine.Accessed June 11, 2024
- Second coming: The re-emergence and modernization of immunotherapy by vaccines as a component of leprosy control. Future Microbiol. 2018;13:1449-51.
- [CrossRef] [PubMed] [Google Scholar]
- A phase 1 antigen dose escalation trial to evaluate safety, tolerability and immunogenicity of the leprosy vaccine candidate LepVax (LEP-F1 + GLA-SE) in healthy adults. Vaccine. 2020;38:1700-7.
- [CrossRef] [PubMed] [Google Scholar]







