Translate this page into:
Lichen Planus Severity Index: A new, valid scoring system to assess the severity of cutaneous lichen planus
Correspondence Address:
Harsimran Kaur
Krishna Institute of Medical Sciences, Karad, Maharashtra
India
| How to cite this article: Kaur H, Nikam BP, Jamale VP, Kale MS. Lichen Planus Severity Index: A new, valid scoring system to assess the severity of cutaneous lichen planus. Indian J Dermatol Venereol Leprol 2020;86:169-175 |
Abstract
Introduction: Lichen planus is a chronic autoimmune inflammatory disorder. At present, there is a lack of any specific scoring system to judge the severity of cutaneous lichen planus. Hence, a study was undertaken to establish and validate a system to define the severity of cutaneous lichen planus, i.e. Lichen Planus Severity Index.
Materials and Methods: Setting: Skin outpatient department, Krishna Institute of Medical Sciences, Karad.
Model: The formulation model was Psoriasis Area Severity Index (PASI) and the validation model was Onychomycosis Severity Index (OSI).
Participants: The consensus group included two dermatologists and two dermatology residents with special interest in lichen planus and a statistician. Results of the consensus group were compared with a preliminary reproducibility group of two dermatologists and four dermatology residents. Later, reliability assessment was carried out by two groups: 1. Twenty-one dermatologists scored 20 photographs of four patients of lichen planus after being trained to use Lichen Planus Severity Index. 2. Six doctors (three experts and three randomly selected physicians) evaluated ten real-world patients of lichen planus in skin outpatient department. The physicians were blind to the scores assigned by experts.
Steps to Calculate Score: There are five morphological types of lesions seen in lichen planus, namely, erythematous papule, violaceous papule, violaceous plaque, hyperpigmented hypertrophic papule and plaque and postinflammatory hyperpigmentation. Total involved body surface area is determined and a body surface area factor is assigned. Area involvement factor for each of these morphological lesions is calculated and multiplied with the respective multiplication factor. Sum of all the products gives the lesion severity score. Product of lesion severity score with the body surface area factor gives the final Lichen Planus Severity Score.
Results: There was no significant difference between the scores of consensus group and preliminary reproducibility group. Both assessment groups showed high reliability. (Group 1: Cronbach alpha = 0.92, ICC = 0.85; Group 2: Cronbach's alpha = 0.99, ICC = 0.92). The correlation between Lichen Planus Severity Index and the standard Physician Global Assessment score was found to be positive (correlation coefficient = 0.73).
Limitations: The system is tedious and requires a steep learning curve. Possible uses of Lichen Planus Severity Index are yet to be explored and validated.
Conclusion: Lichen Planus Severity Index is a new reproducible tool to grade the severity of lichen planus.
Introduction
Cutaneous lichen planus occurs as bilateral and relatively symmetric lesions on flexor surfaces of the extremities. It is defined as a subacute or chronic dermatosis characterized by small, flat-topped, shiny, polygonal violaceous papules that may coalesce into plaques.[1],[2] Indian studies report a majority cases between 20 and 39 years of age[3] and an incidence of 0.38%–1.4% in a routine dermatology outpatient department.[4]
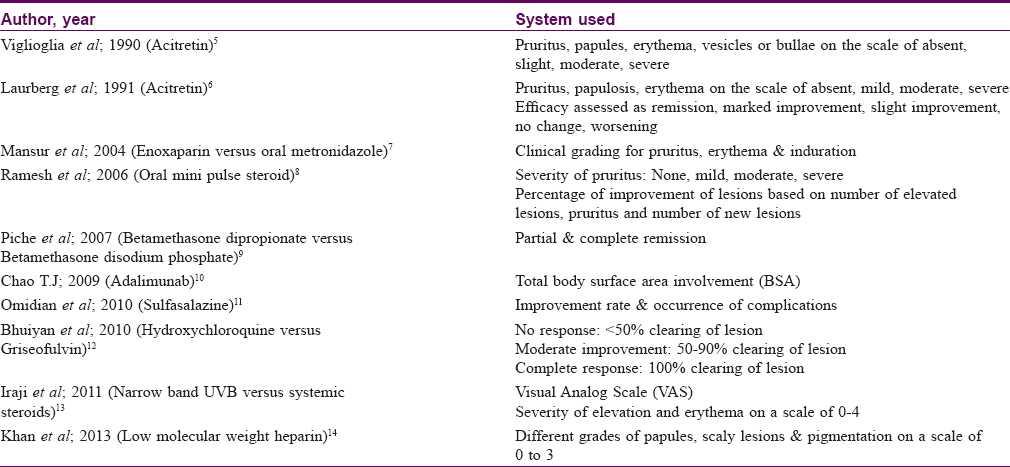
After a thorough search of literature, we were unable to find an effective scoring system to determine the severity of cutaneous lichen planus. Lack of a specific scoring system makes it difficult to decide the adequate dose of various drugs used for treating lichen planus. It also makes the assessment of patient improvement difficult in clinical trials. Most studies use a subjective scale based on erythema and infiltration and a ten-point visual analog scale for pruritus. Scoring systems used by various studies are summarized in [Table - 1].[5],[6],[7],[8],[9],[10],[11],[12],[13],[14]
Materials and Methods
Formulation of the scoring system
A new scoring system was formulated and tried by a group of four dermatologists. It has been named as the Lichen Planus Severity Index. The formulation model for Lichen Planus Severity Index is the Psoriasis Area and Severity Index. Psoriasis Area and Severity Index is based on two variables, namely, area and disease severity. It measures disease severity in terms of erythema, induration and scaling. Likewise, Lichen Planus Severity Index also gives due importance to the area of involvement and disease severity. Disease severity in lichen planus is indicated by its natural history and morphologic evolution of lesions.
Natural history and morphology of lichen planus
Acute eruptive lichen planus usually begins as erythematous papules which progress and turn violaceous. Few of these lesions may further coalesce to form plaques that may be violaceous or even hypertrophic hyperpigmented type. Lesions resolve with classic postinflammatory hyperpigmentation [Figure - 1].
 |
| Figure 1: Natural history of cutaneous lichen planus |
Definitions of morphological variants of lichen planus considered in the scoring system
Erythematous papule
A solid, elevated, flat-topped, circumscribed, reddish, glistening lesion less than 0.5cm in diameter. Erythematous papules are the de novo or precursor lesions of acute eruptive lichen planus and are associated with intense pruritus.[15]
Violaceous papule
A solid, elevated, circumscribed, flat-topped, violaceous lesion with a glistening surface and less than 0.5 cm in diameter. Violaceous papules are the classic pathognomonic lesions described in the definition of lichen planus as “purple, planar, polygonal, pruritic papules.”Well-developed violaceous papules may have a thin, adherent, lacy, whitish scale referred to as the “Wickham striae”.[15]
Violaceous plaque
A solid, violaceous, flat-topped, plateau-like lesion that occupies a larger surface area in comparison with its height above the normal skin level and has a diameter larger than 0.5cm.[15]
Hypertrophic, hyperpigmented papule or plaque
This is the most pruritic and chronic variant of cutaneous lichen planus. Lesions are elevated, violaceous or reddish brown in color (hyperpigmented in Indian skin) with a hyperkeratotic or verrucous surface. Lesions may show accentuated and elevated follicular induration and a chalk-like scale.[15]
Postinflammatory hyperpigmentation
The lesion may be a macule (<0.5 cm) or a patch (>0.5cm). It is flat, hyperpigmented in comparison to the surrounding skin and with the surface level with the surrounding skin. Postinflammatory hyperpigmentation is asymptomatic and indicates disease inactivity or resolution at the affected site.[15]
Calculation of Lichen Planus Severity Index
Step 1: Assigning a body surface area factor
Total involved body surface area is calculated using the Wallace rule of nines.[16]
Because lesions of lichen planus are usually small and discrete, the skip areas are excluded.
For example, if 50% of the abdomen area is involved, it is taken as a 4.5% involvement. By Wallace's rule of 9s, abdomen accounts for 9% of body surface area (half of anterior trunk); so, 50% of abdomen would then be 4.5%. A score ranging from 1 to 5 is assigned based on the percentage of body involvement. This score is the body surface area factor. Hence, the body surface area factor for 1%–20%, 21%–40%, 41%–60%, 61%–80% and 81%–100% body surface area involvement is 1, 2, 3, 4 and 5, respectively.
Step 2: Lesion count and percentage
Total number of lesions are counted followed by counting the total number of erythematous papules, violaceous papules, violaceous plaques, hyperpigmented hypertrophic papules and plaques and postinflammatory hyperpigmentation. Individual percentage of each of these lesions is determined.
Step 3: Assigning area involving factor
Area involvement factor is measured as per the above percentage for each morphological type of lesion. So, if percentage of a lesion is 0%–25% of total body surface area involved, its area involvement factor will be 1. Likewise for 26%–50%, 51%–75% and 76%–100%, area involvement factor will be 2, 3 and 4, respectively.
Step 4: Multiplication factor
Severity of the disease is assessed by natural history and morphology of lesions. Hypertrophic lesions are regarded as the most severe and are assigned the maximum value while postinflammatory hyperpigmentation is the treated inactive form, so, has the minimum value. Multiplication factor used for each morphological type of lesion is as follows:
- Hyperpigmented hypertrophic papules and plaques (Hp) – 4
- Violaceous flat plaques (Vpl) – 3
- Violaceous flat papules (Vp) – 2
- Erythematous papules (Ep) – 1
- Postinflammatory hyperpigmentation (PIH) – 0
Step 5: Final Lichen Planus Severity Index calculation
- Respective area involvement factor (AIF) and multiplication factor (MF) of each of the lesions is multiplied:
A = AIF Hp × MF Hp
B = AIF Vpl × MF Vpl
C = AIF Vp × MF Vp
D = AIF Ep × MF Ep
E = AIF PIH × MF PIH
- The products are added and the final sum is multiplied with body surface area (BSA) factor obtained in Step 1
Final LPSI = (A + B + C + D + E) × BSA factor
Formula for LPSI: {(AIF Ep × MF Ep) + (AIF Vp × MF Vp) + (AIF Vpl × MF Vpl) + (AIF Hp × MF Hp) + (AIF PIH × MF PIH)} × BSA factor
Range of Score
- If a patient is fully cured and all lesions have been converted to PIH, then
PIH = 100%; then AIFPIH= 4, MFPIH= 0
A = 0; B = 0, C = 0, D = 0
E = 4 × 0 = 0
Final LPSI = E × BSA factor = 0 (Minimum score)
- If all lesions in a patient are hypertrophic, hyperpigmented type
Hp = 100%; then AIF Hp = 4, MF Hp = 4
A = 0; B = 0; C = 0; E = 0
D = AIF Hp × MF Hp = 4 × 4 = 16
Final Lichen Planus Severity Index = D × Maximum BSA factor = 16 × 5 = 80 (Maximum Score)
Hence, the final Lichen Planus Severity Index may range from 0 to 80. Practically, in very severe cases, lichen planus is found to affect only 70%–80%body surface area, hence, the maximum score may be 64 (16 × 4).
Steps of LPSI calculation are outlined in [Figure - 2].
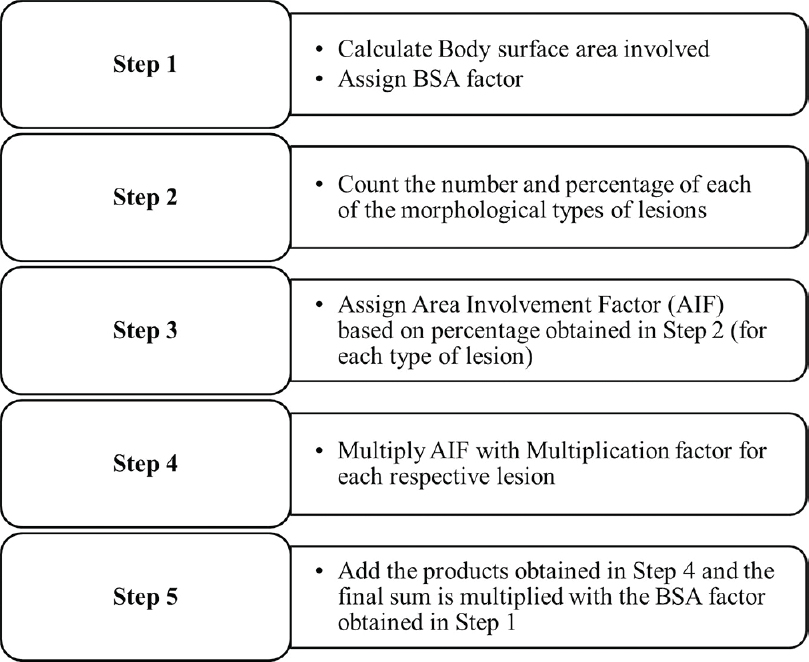 |
| Figure 2: Stepwise calculation of Lichen Planus Severity Index |
Example case
Assuming the red dots are lesions of lichen planus [Figure - 3]a, [Figure - 3]b, [Figure - 3]c, [Figure - 3]d, the scoring system can be applied as follows:
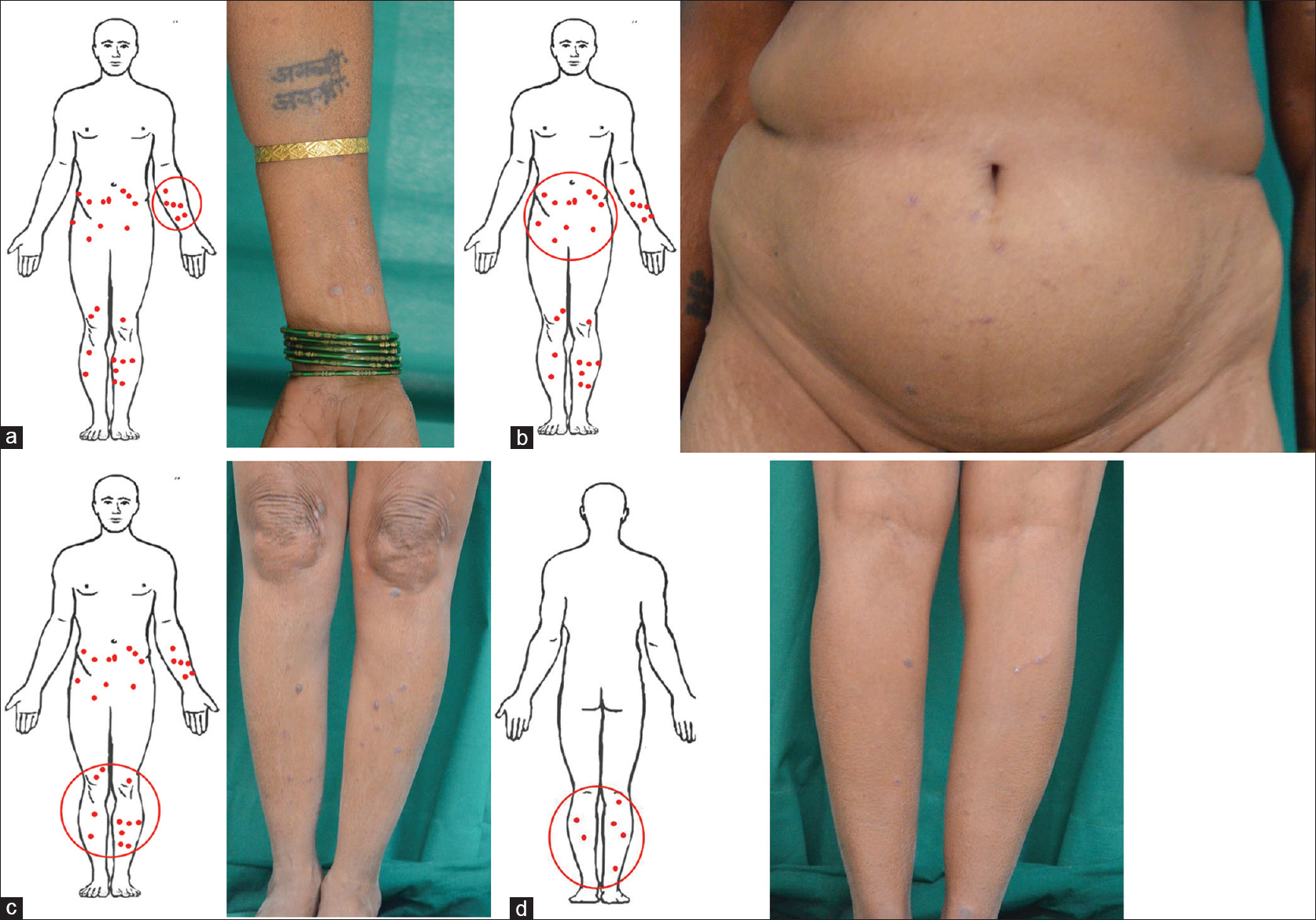 |
| Figure 3: Total number and percentage of erythematous papules, violaceous papules, violaceous plaques, hypertrophic, hyperpigmented papules and plaques and postinflammatory hyperpigmented lesions are determined. (a) left arm, (b) abdomen, (c) lower legs anterior view, (d). lower legs posterior view |
- As less than 20% body surface area is involved, BSA factor is 1
- Calculate
- Total number of
-
- Erythematous papules: 13
- Violaceous papules: 10
- Violaceous plaques: 9
- Hyperpigmented hypertrophic papules and plaques: 5
- Postinflammatory hyperpigmentation: 2
- Total number of lesions: 39
- Individual percentage of each of the above lesions
-
- Erythematous papules: 33.75%
- Violaceous papules: 25.64%
- Violaceous plaques: 23.07%
- Hyperpigmented hypertrophic papules and plaques: 12.32%
- Post inflammatory hyperpigmentation: 5.12%
- Determine area involvement factor for each lesion based on the above percentages on a scale of 0 to 4
Area involvement factor for each lesion
- AIF Ep: 2
- AIF Vp: 2
- AIF Vpl: 1
- AIF Hp: 1
- AIF PIH: 1
- Multiplication factor for each lesion
- MF Ep: 1
- MF Vp: 2
- MF Vpl: 3
- MF Hp: 4
- MF PIH: 0
- Multiply multiplication factor with the area severity factor for each of these lesions and then total
(AIF Ep × MF Ep) + (AIF Vp × MF Vp) + (AIF Vpl × MF Vpl) + (AIF Hp × MF Hp) + (AIF PIH × MF PIH)= (2 × 1) + (2 × 2) + (1 × 3) + (1 × 4) + (1 × 0) =13
- Multiply the final value with the BSA factor to obtain Lichen Planus Severity Index = 13 × 1 = 13.
Validation of Lichen Planus Severity Index
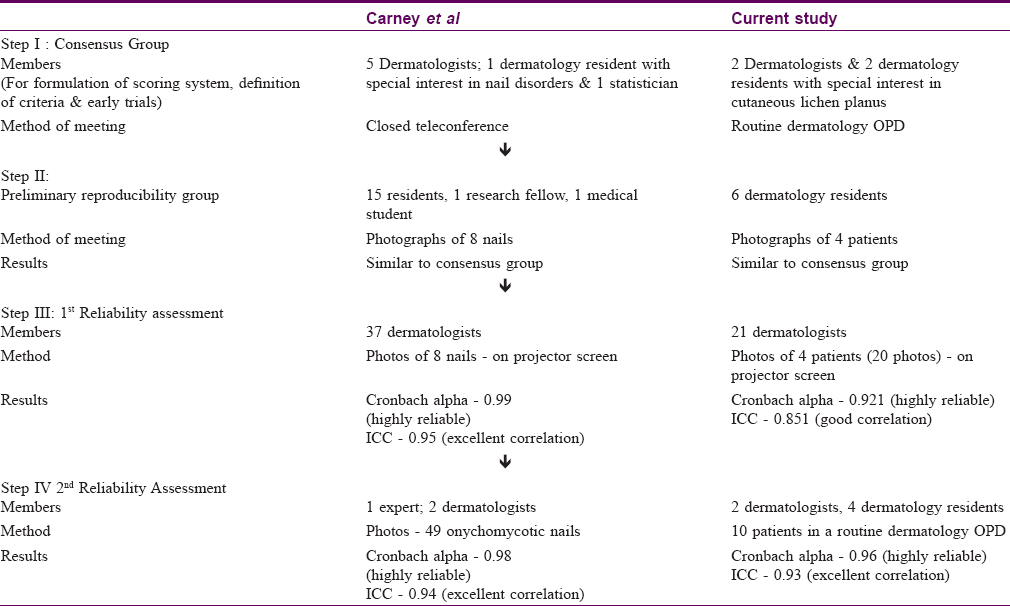
A detailed four-step validation procedure was carried out. Validation model for Lichen Planus Severity Index was Onychomycosis Severity Index validation done by Carney et al.[17]
Participants
The consensus group
The group consisted of two dermatologists and two dermatology residents with special interest in lichen planus. Based on the Psoriasis Area and Severity Index as a model scoring system, this group developed the Lichen Planus Severity Index and implemented it on four patients of lichen planus. One-way analysis of variance test was used to determine the statistical difference between mean scores.
A preliminary reproducibility assessment was conducted by asking six dermatology residents to evaluate the photographs of four patients of lichen planus. The residents recorded their scores on a self-explanatory sheet.
Two assessments were conducted to show the reliability of the scoring system. Reliability was assessed using Cronbach's α and intraclass correlation coefficient using the SPSS-20 software (Statistical Package for the Social Sciences - IBM Corporation, Chicago).
The first reliability assessment included 21 dermatologists who were asked to evaluate the photographs of four patients of lichen planus after being taught how to use the Lichen Planus Severity Index. There was no particular criterion of selection for the evaluators; all dermatologists in the city were invited at a venue, of which 21 turned up. A standard scoring sheet was provided to each dermatologist, and the photographs were projected on a screen for evaluation. The pictured cases included a wide range of morphological differences and disease severity.
The second assessment included two dermatologists and four dermatology residents who scored ten patients of cutaneous lichen planus in a routine dermatology outpatient department. All members of this group were blind to the scores assigned by the other evaluators. Only one or two patients were covered in a day. They were requested to come at a particular time for the rating when the outpatient department was closed. Assessment and evaluation were done after obtaining due consent of the patients.
Correlation with existing scoring system
As Lichen Planus Severity Index is an objective method of assessment, the consensus group attempted to study its correlation with Physician Global Assessment score in 62 patients of cutaneous lichen planus. Physician Global Assessment is a general objective scoring system that describes disease activity, and is scored from 0 to 6.
Score 0 implies clear, 1 almost clear, 2 mild, 3 mild to moderate, 4 moderate, 5 moderate to severe and 6 severe disease activity. Pearson correlation coefficient was used to determine the statistical correlation between Lichen Planus Severity Index and Physician Global Assessment.
Results
Difference between the scores of members of the consensus group was statistically insignificant (P value > 0.434). [Table - 2] shows the mean scores of consensus group in four patients.

Six dermatology residents reproduced scores identical to the consensus group in the subsequent preliminary reproducibility assessment.
Statistical analysis of the mean scores given by 21 doctors found the system to be highly reliable with a good correlation (Cronbach's α = 0.92; intraclass correlation coefficient = 0.85).
In the second reliability assessment conducted with six doctors and ten patients, again, the system proved to be highly reliable with an even better correlation coefficient (Cronbach's α = 0.96; intraclass correlation coefficient = 0.96).
[Table - 3] gives the stepwise summary of members, method of meeting and results of each assessment.
Pearson correlation was assessed between Lichen Planus Severity Index and Physician Global Assessment for 62 patients of cutaneous lichen planus. The relation was found to be linear with an r-value of 0.73 at 95% confidence interval (P value < 0.001) suggestive of a significant positive correlation between the two variables. Further, patients with Physician Global Assessment score of more than 3 were observed to have Lichen Planus Severity Index of more than 40.
Discussion
Our results demonstrate excellent interobserver and intraobserver reliability. Though there were minor interobserver variations, probably due to incorrect identification of morphological types of lesions, there was no significant difference in the final scores allotted by different observers. This is because the system is not based on individual number of lesions but on area involvement factor that accounts for a range of percentage number of lesions.
If all lesions are postinflammatory hyperpigmentation, score drops down to zero (a state where patient is asymptomatic and comfortable). More the extent of involvement, more the score, but also, more the severity (hypertrophic, slow to respond disease), more the score. Hence, the system gives an idea of both qualitative and quantitative disease burden. As patients with clinically evident moderate to severe disease were found to have Lichen Planus Severity Index of more than 40, the consensus group concluded that cases with Lichen Planus Severity Index of less than 40 had mild to moderate cutaneous lichen planus, while those with Lichen Planus Severity Index of more than 40 had severe disease. In this context, the tool needs to be explored in the future as an indicator of prognosis.
While members of the consensus group could complete the score assessment in 3–5 minutes, it was observed that the mean duration for beginners was 8–10 minutes. The scoring system is tedious with a steep learning curve and there may be interobserver variability; however, these limitations can be overcome with practice and time. When the outpatient department was very busy, the patient was requested to wait back and after his/her consent was obtained, pictures were taken and the calculations done later. With practice, one is usually able to jump to the step of percentage of each type of morphological lesion. After that, the calculation barely takes 1–2 min. Calculations can be made faster with the use of self-explanatory sheets.
The severity and the extent of the lesion usually decide the treatment regimen and dosage required. Lichen Planus Severity Index can be used to assess disease severity but whether it can be used directly to guide drug dosages remains to be evaluated in future studies. Once tested and practiced, it may be used for documentation of disease severity and as an aid to precise clinical discussions.
Conclusion
Lichen Planus Severity Index is a reliable and valid tool to assess the disease severity of cutaneous lichen planus. Its potential as an efficacy determinant needs to be tapped in future clinical trials.
Acknowledgement
Kolhapur Dermatology Association for participation in reliability assessments.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Aly DG, Shahin RS. Oxidative stress in lichen planus. ActaDermatovenerol Alp PannonicaAdriat 2010;19:3-11.
[Google Scholar]
|
| 2. |
Garg VK, Nangia A, Logani K, Sharma RC. Lichen planus-a clinico-histopathological. Indian J DermatolVenereolLeprol 2000;66:193-5.
[Google Scholar]
|
| 3. |
Haldar S, Khopkar U. Lichen Planus. Clinical features of lichen planus. In 1st ed. India: Jaypee, 2013. Ch. 3: ccclinical features of lichen planus; p. 15-43.
[Google Scholar]
|
| 4. |
Bhattacharya M, Kaur I, Kumar B. Lichen planus: A clinical and epidemiological study. J Dermatol 2000;27:576-82.
[Google Scholar]
|
| 5. |
Viglioglia PA, Villanueva CR, Martorano AD, Cahuepé AM, Traballi CA, Geiger JM, et al. Efficacy of acitretin in severe cutaneous lichen planus. J Am AcadDermatol 1990;22:852-3.
[Google Scholar]
|
| 6. |
Laurberg G, Geiger JM, Hjorth N, Holm P, Hou-Jensen K, Jacobsen KU, et al. Treatment of lichen planus with acitretin. A double-blind, placebo-controlled study in 65 patients. J Am AcadDermatol 1991;24:434-7.
[Google Scholar]
|
| 7. |
Mansur A, Yasar S. A comparison of the efficacy of enoxaparin with oral metronidazole in idiopathic lichen planus. Internet J Dermatol 2004;3:1-7.
[Google Scholar]
|
| 8. |
Ramesh M, Balachandran C, Shenoi SD, Rai VM. Efficacy of steroid oral mini-pulse therapy in lichen planus: An open trial in 35 patients. Indian J DermatolVenereolLeprol 2006;72:156-7.
[Google Scholar]
|
| 9. |
Pitche P, Saka B, Kombate K, Tchangai-Walla K. Treatment of generalized cutaneous lichen planus with dipropionate and betamethasone disodium phosphate: An open study of 73 cases. Ann DermatolVenereol 2007;134:237-40.
[Google Scholar]
|
| 10. |
Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis 2009;84:325-8.
[Google Scholar]
|
| 11. |
Omidian M, Ayoobi A, Mapar MA, Feily A, Cheraghian B. Efficacy of sulfasalazine in the treatment of generalized lichen planus: Randomized double-blinded clinical trial on 52 patients. J EurAcadDermatolVenereol 2010;24:1051-4.
[Google Scholar]
|
| 12. |
Bhuiyan I, Wahab M. Comparative efficacy of hydroxychloroquine and griseofulvin in the treatment of lichen planus. J Pak AssocDermatol 2010;20:79-83.
[Google Scholar]
|
| 13. |
Iraji F, Faghihi G, Asilian A, Siadat AH, Larijani FT, Akbari M, et al. Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial. J Res Med Sci 2011;16:1578-82.
[Google Scholar]
|
| 14. |
Khan MSI, Uddin MN, Shah MOR, Khondekar L, Hasan MS. Efficacy of low dose molecular weight heparin in the treatment of cutaneous lichen planus. J Armed Forces Med Coll Bangladesh 2013;9:2-7.
[Google Scholar]
|
| 15. |
Garg A, Levin NA, Bernhard J.D Structure of skin lesions and fundamentals of clinical diagnosis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. Philadelphia: McGraw-Hill; 2012. p. 26-42.
[Google Scholar]
|
| 16. |
Hettiaratchy S, Papini R. Initial management of a major burn: II – Assessment and resuscitation. BMJ 2004;329:101-3.
[Google Scholar]
|
| 17. |
Carney C, Tosti A, Daniel R, Scher R, Rich P, DeCoster J, et al. Anew classification system for grading the severity of onychomycosis: Onychomycosis severity index. Arch Dermatol 2011;147:1277-82.
[Google Scholar]
|
Fulltext Views
11,295
PDF downloads
4,213





