Translate this page into:
Lichenoid pseudovesicular papular eruption on nose: A papular facial dermatosis probably related to actinic lichen nitidus or micropapular polymorphous light eruption
2 Departments of Pathology, All India Institute of Medical Sciences, New Delhi, India
3 Departments of Dermatology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Saurabh Singh
Dermatology OPD, Main OPD Block, All India Institute of Medical Sciences, Basni Phase-2nd, Jodhpur - 342 005, Rajasthan
India
| How to cite this article: Singh S, Singh A, Mallick S, Arava S, Ramam M. Lichenoid pseudovesicular papular eruption on nose: A papular facial dermatosis probably related to actinic lichen nitidus or micropapular polymorphous light eruption. Indian J Dermatol Venereol Leprol 2019;85:597-604 |
Abstract
Background: Facial papules are a feature of several clinical conditions and may present both diagnostic and therapeutic challenges.
Aim: To describe a grouped papular eruption on the nose and adjoining cheeks that has not been well characterized previously.
Materials and Methods: A series of consecutive patients with a papular eruption predominantly involving nose and cheeks were evaluated, treated and followed up prospectively at tertiary care centers. Demographic details, clinical features, histopathology and response to treatment were recorded.
Results: There were five men and six women (mean age 29.9 ± 6.9 years) who had disease for a mean duration of 17.3 ± 11.1 months. All patients presented with a predominantly asymptomatic eruption of monomorphic, pseudovesicular, grouped, skin colored to slightly erythematous papules prominently involving the tip of nose, nasal alae, philtrum and the adjoining cheeks. A total of 15 biopsies from 11 patients were analyzed and the predominant finding was a dense, focal lymphoid infiltrate restricted to the upper dermis with basal cell damage and atrophy of the overlying epidermis. The eruption ran a chronic course from several months to years.
Limitations: Direct immunofluorescence could not be performed except in one case. Immunohistochemical stains for CD4 and CD8 could not be done owing to nonavailability. Phototesting was undertaken in one patient only.
Conclusion: Small grouped papules on the nose and adjoining skin with a lichenoid histopathology appear to represent a distinct clinicopathological entity. It may be related to actinic lichen nitidus/micropapular variant of polymorphous light eruption.
Introduction
Facial papules may be the presenting feature of several conditions, including inflammatory, photosensitive, infective and granulomatous disorders as well as benign and malignant neoplasms. Lichenoid skin eruptions consist of a clinically heterogeneous group, which is more or less unified by histopathological similarities. Some of the papular lichenoid eruptions that affect face include lichen planus, actinic lichen planus, lichen nitidus and rarely, lichenoid drug eruption and discoid lupus erythematosus. These entities may be confused with other papular facial disorders such as rosacea, discoid lupus erythematosus, granulosis rubra nasi, granulomatous perioral and facial dermatitis, acne agminata, sarcoidosis, Jessner's lymphocytic infiltrate, papular solar elastosis and rarely, polymorphous light eruption. The differentiation between these entities requires clinical, histopathological and laboratory evaluation. Over the past few years, we have recorded a series of patients presenting with a relatively asymptomatic tiny pseudovesicular papular eruption involving the nose and adjoining areas that predominantly showed a lichenoid infiltrate on biopsy. This condition does not fit well into the previously described facial papular disorders or another clinicopathological entity. We present our observations in a series of 11 patients.
Materials and Methods
The first case of this distinctive nasal and facial eruption was seen by the lead author (SS) at AIIMS, Delhi in February 2012. Consecutive patients seen in subsequent years matching the clinical profile were evaluated and followed up at different centers wherever SS worked (AIIMS—Delhi, ESICMCH—Faridabad and AIIMS—Jodhpur). Clinical profiling of the cases was done by SS and/or MR, whereas histopathology findings were noted by all authors. A standard 4 mm punch biopsy was performed on all patients and stained with hematoxylin–eosin, special stains (Alcian blue, periodic acid–Schiff) and immunohistochemical stains (CD3, CD20), as appropriate. The blood investigations performed included complete blood count and peripheral smear, erythrocyte sedimentation rate, serum biochemistry, antinuclear antibody (Hep2 immunofluorescence) and thyroid stimulating hormone (whenever suspected clinically). Other investigations were performed if deemed clinically relevant.
Results
There were six women and five men, and the mean age at presentation was 29.9 ± 6.9 years (21–40 years) and the mean duration of disease was 17.4 ± 11.1 months (3–40 months). All patients had skin type IV and belonged to Northern India (Delhi-NCR, Haryana, Uttar Pradesh and Bihar). The lesions were asymptomatic in eight patients, while three patients complained of increased erythema, sweating and pruritus on exposure to sunlight. There was no history of rosacea or collagen vascular disease or aggravation to food or emotional stimuli. There was no history of hyperhidrosis on nose, insect bite, facial flushing or burning. Drug history and occupation were noncontributory. Four females were homemakers and one was a student. All the males were predominantly indoor office workers.
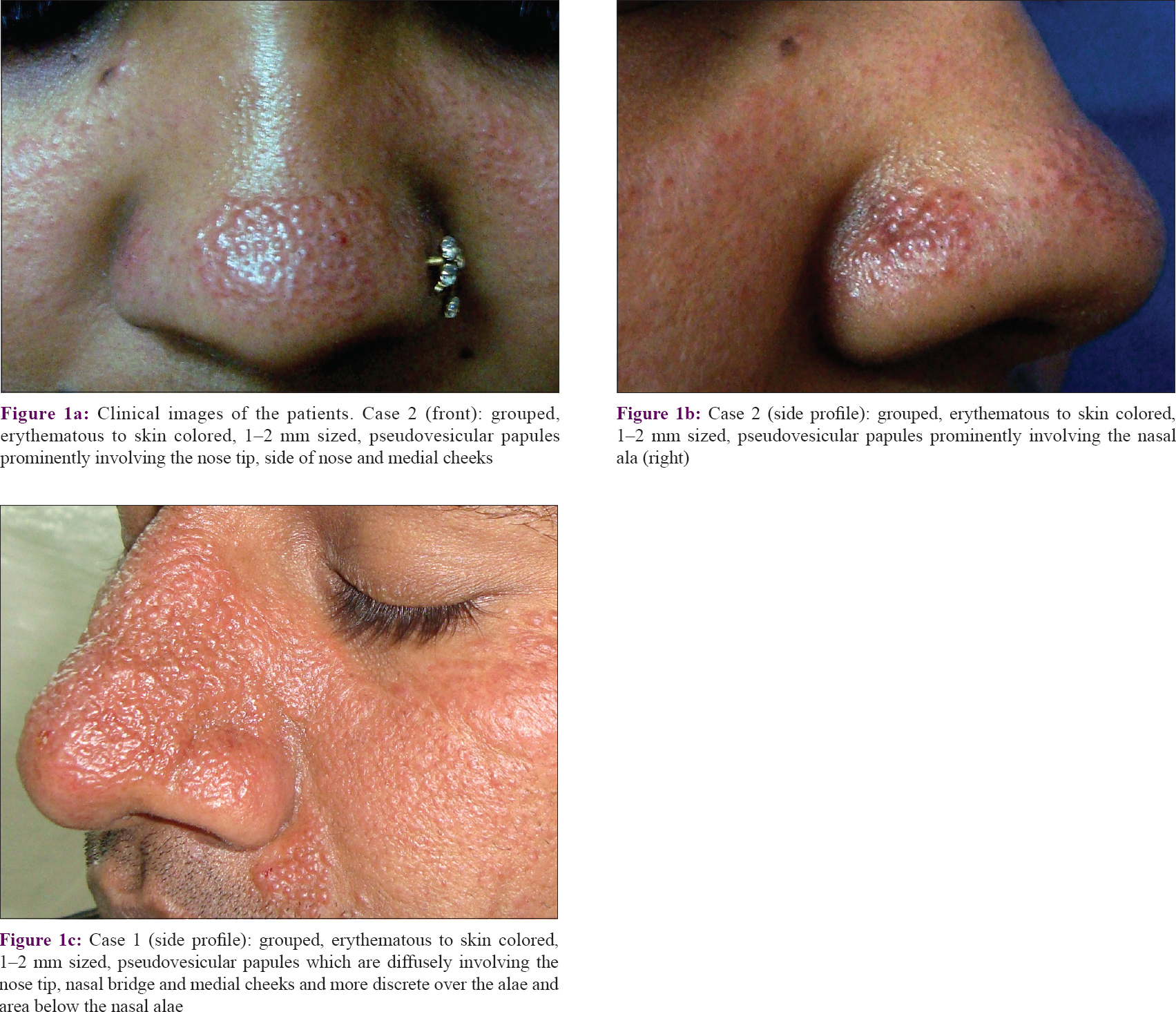
Barring minor differences, the clinical and histopathological findings in all our cases were strikingly similar [Table - 1]. In summary, the lesions started gradually over a few weeks from skin colored micropapular eruption to varying hues of skin colored, erythematous or hyperpigmented grouped papules predominantly involving the nose and adjoining centro-facial region. The representative lesions were 1–2 mm sized, skin colored to erythematous, pseudovesicular papules uniformly involving the nose tip [Figure - 1](a)–(c)]. The other common sites were nasal alae (eight patients), bridge of nose (three patients), cheeks (three patients), below the ala (two patients) and forehead (two patients). Case 1 showed the most extensive eruption with marked involvement of the nose, a diffuse papular infiltration on the cheeks and papules on the forehead [Figure - 1](c)]. Mild background erythema was noticed in four patients. Papules showed predominant hyperpigmentation in three patients. Other associated findings were presence of asymptomatic hypopigmented, grouped, nonkoebnerizing plane-topped papules on the dorsae of hands [Case 1 – [Figure - 2]a and Case 9 –- [Figure - 2]b and forearms [two patients, Cases 4 and 6].
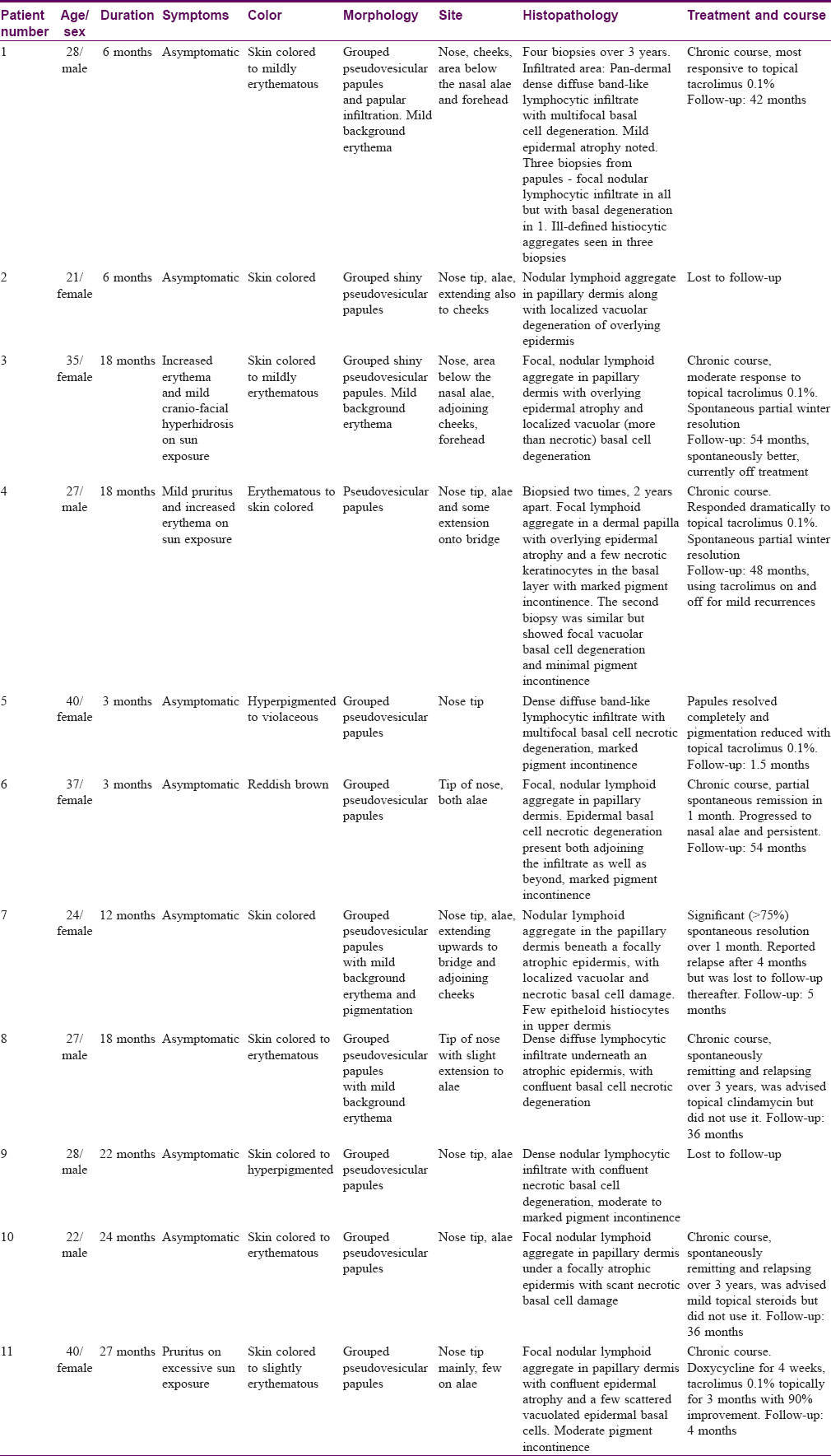
 |
| Figure 1: |
 |
| Figure 2: |
Diascopy did not reveal apple jelly nodules. The clinical differential diagnoses considered were granulosis rubra nasi, eccrine hidrocystoma, rosacea, papular sarcoidosis, discoid lupus erythematosus, idiopathic photodermatoses (actinic lichen nitidus/micropapular polymorphous light eruption), pseudolymphoma and papular elastosis.
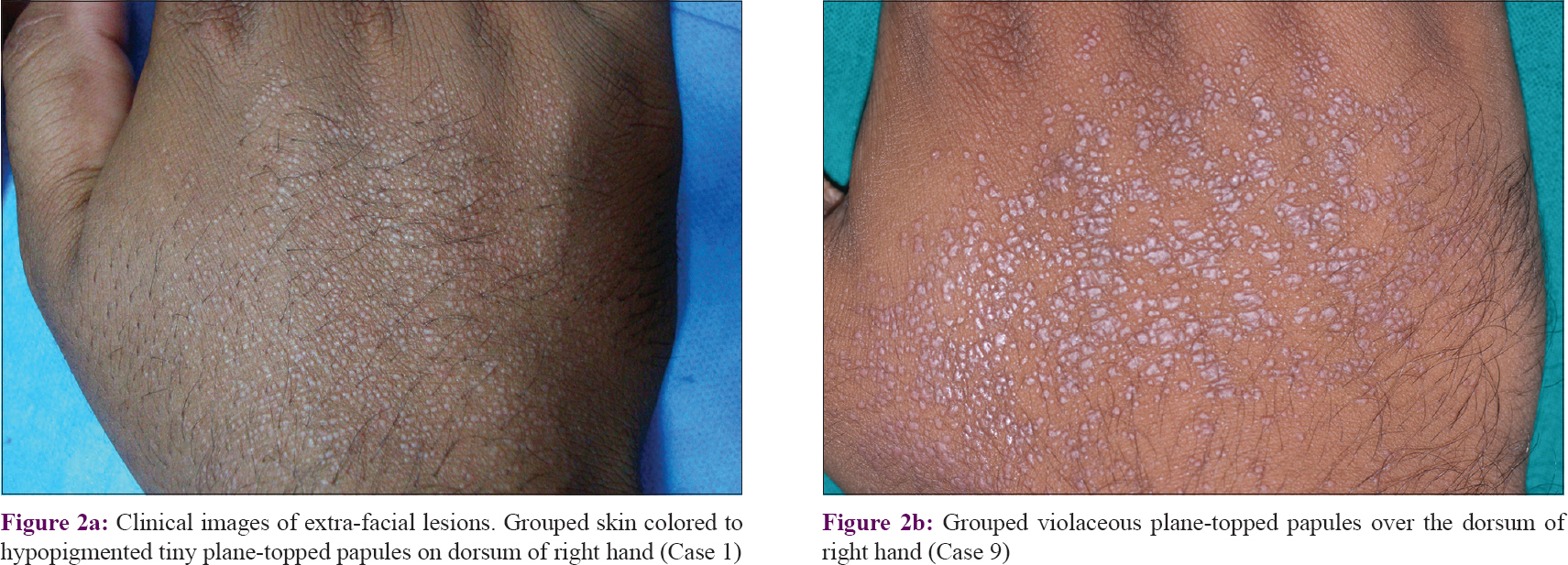
A total of 15 punch biopsies from 11 patients (including four biopsies from patient 1 and two biopsies from patient 4, done at different times) were evaluated. The typical findings were a focal nodular lymphoid aggregate in the papillary/upper dermis (11 biopsies), epidermal basal cell degeneration (13 biopsies, predominantly necrotic in eight and vacuolar in five) and focal epidermal atrophy (seven biopsies). All three findings were seen in seven biopsies. Basal cell degeneration was focal in eight and confluent in five biopsies. Other findings included follicular plugging (12 biopsies), moderate to marked pigment incontinence (11 biopsies) and ill-defined aggregates of epithelioid cells within the infiltrates (six biopsies). Histopathologic findings are summarized in [Figure - 3][(a) to [Figure - 3](b) [Figure - 3](c)–-. Special stains, including Ziehl–Neelsen, Alcian blue and periodic acid–Schiff, were done in all cases and were noncontributory. Immunohistochemistry showed nearly equal numbers of lymphocytes stained with anti-CD3 and anti-CD20 antibodies. Direct immunofluorescence was performed only on one biopsy (patient 4) and did not reveal deposits of any immunoreactants. The papules on the dorsae of hand in two patients were biopsied, and in both patients they revealed features suggestive of lichen nitidus. All blood investigations were within the normal range. In view of the extensive involvement, Case 1 received a diagnosis of chronic actinic dermatitis and underwent phototesting with ultraviolet A (maximum 20 J/cm [2]) and narrowband UVB (maximum 1 J/cm [2]) but showed lack of photosensitivity to these wavelengths. Other patients were not phototested.
 |
| Figure 3: |
Treatment and follow-up of all patients are summarized in [Table - 1]. Overall, nine patients followed up for a mean period of 31.2 ± 21.8 months (1.5–54 months) and two patients were lost to follow-up (patients 2 and 9). In total, five patients showed a tendency for partial/complete spontaneous remission. Case 1 was initially treated with photoprotection, sunscreens and oral hydroxychloroquine 400 mg per day for 3 months without improvement. Topical tacrolimus 0.1% ointment once at night was then added and the patient showed significant improvement after 2 months with complete resolution of erythema, significant flattening of papules on the cheek and forehead, and partial improvement in papules on the nose and adjoining skin [Figure - 4](a) and [Figure - 4](b). Case 3 was initially treated with oral doxycycline 100 mg twice daily along with topical tacrolimus 0.1% and sunscreens for 2 weeks with a mild response. She had 30 to 40% improvement over the next 3 weeks but was then lost to follow-up. She claimed to be significantly better without treatment, when contacted telephonically 7 years later. Case 4 was initially treated with oral hydroxychloroquine 400 mg/day which he took for a year and with mild improvement. On switching to topical tacrolimus 0.1% ointment once at night, he reported near-complete resolution in 3 weeks. He continues to use topical tacrolimus intermittently for mild flares.
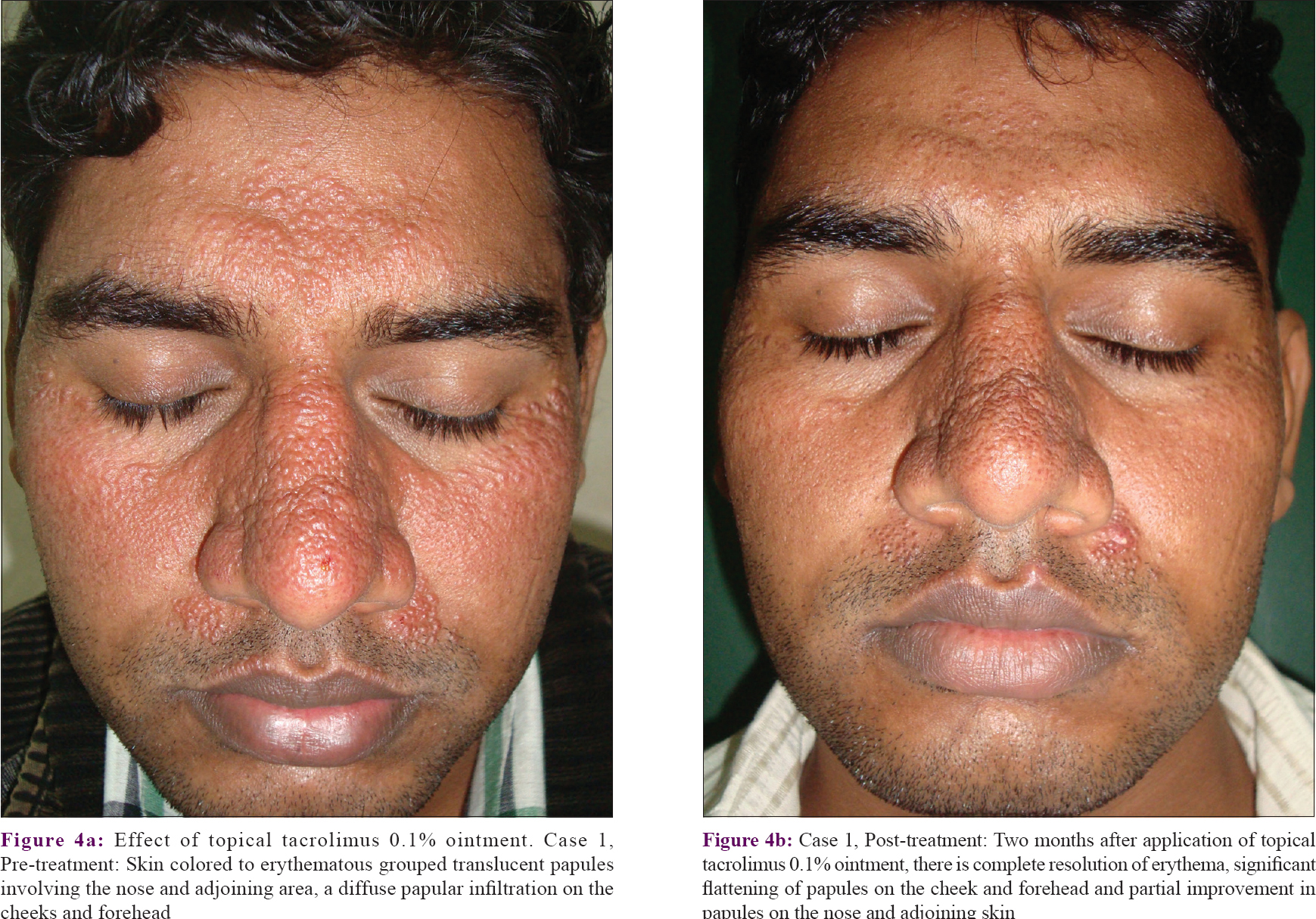 |
| Figure 4: |
Discussion
The eruption in our patients appears distinct from other facial dermatoses which were considered in the differential diagnosis [Table - 2], including granulosis rubra nasi and papular variants of rosacea, sarcoidosis and pseudolymphomas.
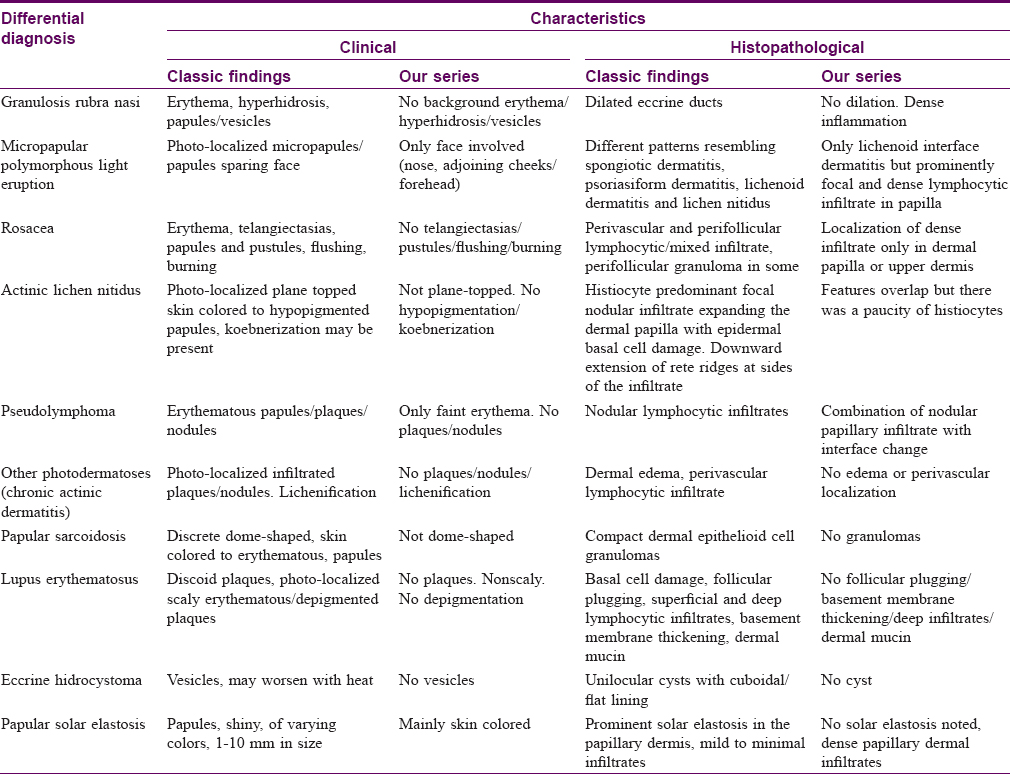
Granulosis rubra nasi is characterized by tiny papules/vesicles and erythema prominently on the nose and hence closely resembles the eruption in our patients. It has a peak incidence in younger children and usually resolves at puberty, but this was not the case in our series. However, none of our patients reported nasal hyperhidrosis which is uniformly associated with granulosis rubra nasi.[1] Besides, biopsy of granulosis rubra nasi shows a minimal inflammatory infiltrate, unlike the dense lichenoid infiltrates in our cases. Granulomatous and papular variants of rosacea have overlapping clinical features in the form of small papules on the facial convexities, often accompanied by erythema and telangiectasias. Some patients have predominant nasal involvement. However, rosacea shows a moderate, follicle-centered infiltrate of lymphocytes and histiocytes and/or small epithelioid granulomas and granulomatous rosacea is primarily a histological variant.[2] Lichenoid infiltrates, as seen in our patients, are not a feature of any form of rosacea. The recently described miliarial-type perifollicular B-cell pseudolymphoma presents with tiny papules but these are scattered on the face and do not have a preference for the nose or central face. Biopsy reveals perifollicular germinal centers without epidermal changes.[3] Jessner's lymphocytic infiltrate also shows a facial papular eruption but is often asymmetric and is characterized more commonly by plaque-type lesions situated away from the nose.[4] Histopathology shows a predominant perivascular lymphoid infiltrate without epidermal changes, unlike the findings in our patients.[4] Sarcoidosis, eccrine hidrocystoma, discoid lupus erythematosus and papular solar elastosis were easily excluded on histopathology.
Other authors appear to have seen similar cases. One previous report described a 16-year-old boy with a diffuse papular infiltration on the nose and cheeks and hyperhidrosis of the nose tip for 5 years. The accompanying high-power photomicrograph shows a moderately dense lymphocytic infiltrate in the dermis. A diagnosis of granulosis rubra nasi was made but there was no comment on the histopathological finding, which is unlike that seen in this condition.[1] Two other reports appear to be of the same woman, described at different ages by different authors from the same city.[5],[6] These articles describe a young woman with a longstanding, photosensitive eruption composed of erythematous vesicles on the cheeks, nose and forehead. Biopsy revealed a moderate to dense nodular lymphoid aggregate mainly in the upper dermis. The first report labeled the condition granulosis rubra nasi, whereas the second report labeled it as a T-cell predominant cutaneous lymphoid hyperplasia. The clinical and histopathological appearance in all these three reports is similar to those noted in our patients and we believe they may represent the eruption we have described in this paper.
A micropapular variant of polymorphous light eruption has been described in darker skin types and has been reported under different names.[7],[8],[9],[10],[11],[12] The histopathological spectrum of this eruption includes a lichenoid dermatitis resembling lichen nitidus, a spongiotic dermatitis and a psoriasiform dermatitis. Although it uniformly spares the face, the clinical and histopathological appearance and the skin phototype of affected patients resembles that in our patients. Actinic lichen nitidus is characterized by classic lesions of lichen nitidus developing predominantly on photo-exposed sites and may show koebnerization as in classic lichen nitidus. Histologically, there is a focal lichenoid infiltrate with admixture of histiocytes and giant cells indistinguishable from lichen nitidus.[13] Some authors believe actinic lichen nitidus is a variant of micropapular polymorphous light eruption described as summertime actinic lichenoid eruption,[7],[14] while others believe that it is an independent entity.[9]
Some clinical and histopathological resemblance to actinic lichen nitidus was evident in our patients though histiocytes were not prominent in the infiltrate and the pseudovesicular nature of eruption was striking. These features would be unusual for actinic lichen nitidus. Despite this, we wonder if the eruption in our patients represents a pseudovesicular variant of either actinic lichen nitidus or facial micropapular polymorphous light eruption in view of the grouped shiny photolocalized papular eruption. Further studies may resolve this dilemma but a descriptive term such as lichenoid pseudovesicular papular eruption of the nose may help to categorize this group of patients and facilitate their recognition and study by other workers till its nosological position is settled.
The exact etiopathogenesis of the eruption remains obscure. The reason for the pseudovesicular appearance of the lesions is unclear but may be possibly because of the overlying epidermal atrophy, which is a feature of other translucent lesions such as sarcoidosis and eccrine hidrocystoma. Sunlight seemed to play a role in some of our patients: three patients had photosensitive erythema and pruritus, two patients had spontaneous partial remission in winters, whereas two patients had asymptomatic lichen nitidus-like lesions on the dorsae of both hands. Geographical location or occupation did not appear to play a role in our patients and there was no association with drug intake.
Our report has some limitations. The length of follow-up was relatively short in some patients. Direct immunofluorescence to look for immunoreactant deposition was performed in only one patient and phototesting was also undertaken in one patient only. Immunohistochemical stains for CD4 and CD8 could not be done owing to nonavailability.
Conclusion
We describe a distinctive pseudovesicular, monomorphic micropapular eruption predominantly involving the nose and adjoining cheeks that affects young to middle-aged people with no gender predilection and may be photoaggravated in some cases. It runs a chronic course and responds moderately well to topical tacrolimus. We propose the term lichenoid pseudovesicular papular eruption of the nose for this condition.
Acknowledgment
Dr. Sudhir Singh (ex-senior resident) and Dr. Suman Patra (senior resident), Department of Dermatology, AIIMS, Delhi and Dr. Shanta Passi, Dermatologist at ESIC Medical College and Hospital, Faridabad for helping with collection of cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sonthalia S, Singal A, Sharma R. Hyperhidrosis, vesicles, and papules over the nose: Granulosis rubra nasi. Indian J Dermatol Venereol Leprol 2012;78:97-8.
[Google Scholar]
|
| 2. |
Sanchez JL, Berlingeri-Ramos AC, Dueno DV. Granulomatous Rosacea. Am J Dermatopathol 2008;30:6-9.
[Google Scholar]
|
| 3. |
Moulonguet I, Ghnassia M, Molina T, Fraitag S. Miliarial-type perifollicular B-cell pseudolymphoma (lymphocytoma cutis): A misleading eruption in two women. J Cutan Pathol 2012;39:1016-21.
[Google Scholar]
|
| 4. |
Engin B, Songür A, Kutlubay Z, Serdaroǧlu S. Lymphocytic infiltrations of face. Clin Dermatol 2014;32:101-8.
[Google Scholar]
|
| 5. |
Kumar P, Gosai A, Mondal AK, Lal NR, Gharami RC. Granulosis rubra nasi: A rare condition treated successfully with topical tacrolimus. Dermatol Reports 2012;4:e5.
[Google Scholar]
|
| 6. |
Jain A, Majumdar B, Sen D, Sen S, Mishra P, Samanta A. Asymptomatic papules over central and pericentral areas of the face. Indian Dermatol Online J 2015;6:198-200.
[Google Scholar]
|
| 7. |
Bedi TR. Summertime actinic lichenoid eruption. Dermatologica 1978;157:115-25.
[Google Scholar]
|
| 8. |
Horio T, Danno K, Furukawa F, Okamoto H. Micropapular light eruption. Nihon Hifuka Gakkai Zasshi 1986;96:519-22.
[Google Scholar]
|
| 9. |
Hussain K. Summertime actinic lichenoid eruption, a distinct entity, should be termed actinic lichen nitidus. Arch Dermatol 1998;134:1302-3.
[Google Scholar]
|
| 10. |
Kontos AP, Cusack CA, Chaffins M, Lim HW. Polymorphous light eruption in African Americans: Pinpoint papular variant. Photodermatol Photoimmunol Photomed 2002;18:303-6.
[Google Scholar]
|
| 11. |
Chiam LY, Chong WS. Pinpoint papular polymorphous light eruption in Asian skin: A variant in darker-skinned individuals. Photodermatol Photoimmunol Photomed 2009;25:71-4.
[Google Scholar]
|
| 12. |
Shah N, Khaitan BK, Ramam M, Sharma VK, Singh MK. Photosensitive spongiotic/lichenoid eruption of micropapules and plaques: A morphologically distinct entity. Indian J Dermatol Venereol Leprol 2013;79:497-505.
[Google Scholar]
|
| 13. |
Glorioso S, Jackson SC, Kopel AJ, Lewis V, Nicotri T Jr. Actinic lichen nitidus in 3 African American patients. J Am Acad Dermatol 2006;54:S48-9.
[Google Scholar]
|
| 14. |
Isaacson D, Turner ML, Elgart ML. Summertime actinic lichenoid eruption (lichen planus actinicus). J Am Acad Dermatol 1981;4:404-11.
[Google Scholar]
|
Fulltext Views
17,417
PDF downloads
3,448





