Translate this page into:
Localised cutaneous siderosis following intravenous ferric carboxymaltose infusion: An underreported complication
Corresponding author: Dr. Vishal Gaurav, Department of Dermatology and Venereology, Pt. Madan Mohan Malaviya Hospital, New Delhi, India. mevishalgaurav@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Misri R, Sharma S, Gaurav V. Localised cutaneous siderosis following intravenous ferric carboxymaltose infusion: An underreported complication. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_542_2025
Dear Editor
Iron deficiency anaemia (IDA) is a common comorbidity during pregnancy, affecting nearly 36.8% of expectant mothers.1 It is linked to several maternal and fetal adverse outcomes, such as preterm labour, low birth weight, and increased risk of peripartum and postpartum haemorrhage requiring blood transfusion.1 Early detection and appropriate management of IDA is crucial for ameliorating such risks. Ferric carboxymaltose, an intravenous (IV) iron formulation, is frequently employed for rapid replenishment of iron levels, particularly in pregnant women who are intolerant or unresponsive to oral iron supplementation. IV iron is safe during the second and third trimesters and is more effective at restoring iron stores than oral iron, with a reduced frequency of adverse gastrointestinal effects.2
A 28-year-old gravida 2 para 1 woman presented with persistent hyperpigmentation over her left forearm and dorsum of the left hand. [Figures 1a and 1b], It developed following an IV infusion of 1000 mg ferric carboxymaltose for pregnancy-related IDA 5 weeks ago. At the time of infusion, she was in her second trimester suffering from anaemia, evidenced by a haemoglobin level of 9.4 g/dL (94 g/L) and laboratory parameters indicative of iron deficiency, including a reticulocyte count of 78 × 10⁹/L, serum iron of 7.3 µmol/L, ferritin of 15 µg/L, and transferrin saturation of 13%. She initially received oral ferrous fumarate but developed severe diarrhoea, necessitating a switch to IV iron therapy. The infusion was administered via a vein on the dorsum of the left hand in a dose of 1000 mg diluted in 100 mL of normal saline, delivered at an initial rate of 15 mL/hour over 20 minutes, and increased to 120 mL/hour thereafter. Dermoscopy revealed perifollicular and perieccrine brown deposits [Figure 2]. Histopathology revealed abundant brown pigment around capillaries and eccrine glands [Figures 3a and 3b], confirmed as haemosiderin with Perl’s stain [Figure 3c]. The patient was reassured about the benign nature of the condition and informed about potential treatment options, including laser therapy, to address the hyperpigmentation. However, she elected to pursue conservative management with observation due to pregnancy.
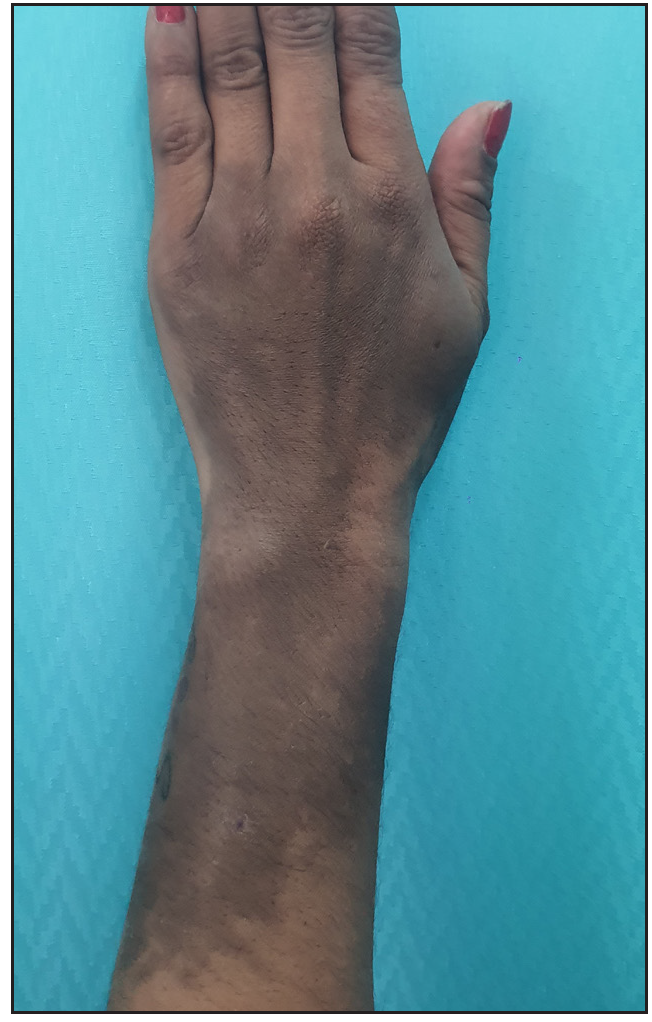
- Well-to-ill-defined slate-gray hyperpigmented patch measuring approximately 10 cm × 6 cm, extending from the dorsum of the mid-forearm and left hand.
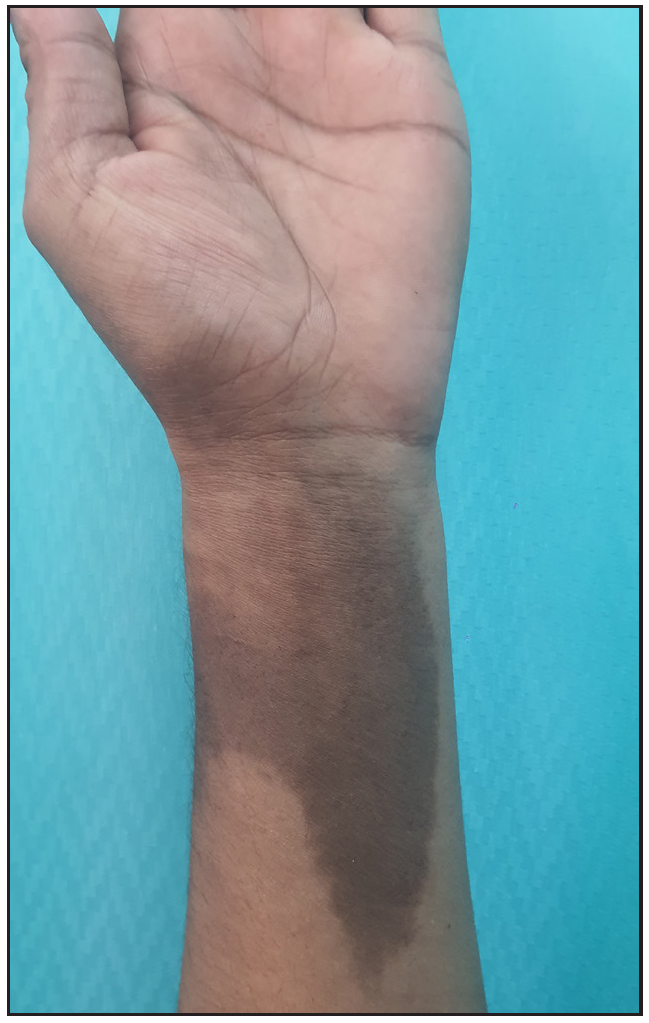
- Well-to-ill-defined slate-grey hyperpigmented patch measuring approximately 10 cm × 6 cm, extending from the ventral aspect of the forearm.
![Dermoscopy [AM7115MZT Dino-Lite Edge 3.0 digital microscope] showing perieccrine (black arrows) and perifollicular (blue arrow) brown deposits (Polarised, 35x).](/content/126/2025/0/1/img/IJDVL_542_2025-g3.png)
- Dermoscopy [AM7115MZT Dino-Lite Edge 3.0 digital microscope] showing perieccrine (black arrows) and perifollicular (blue arrow) brown deposits (Polarised, 35x).
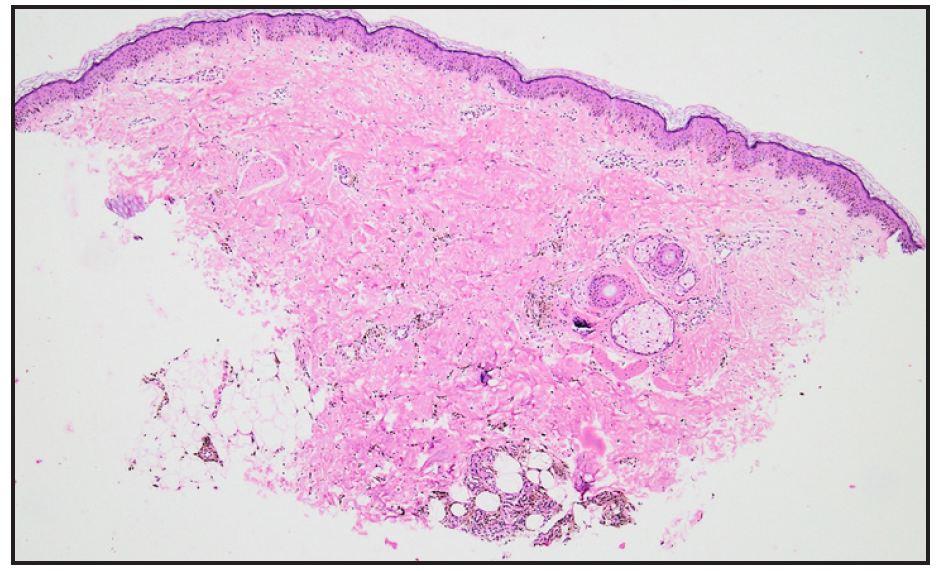
- Photomicrograph showing Mild perivascular inflammation in the dermis along with abundant brown pigment throughout the dermis around capillaries and eccrine.glands (Haematoxylin and eosin, 40x).
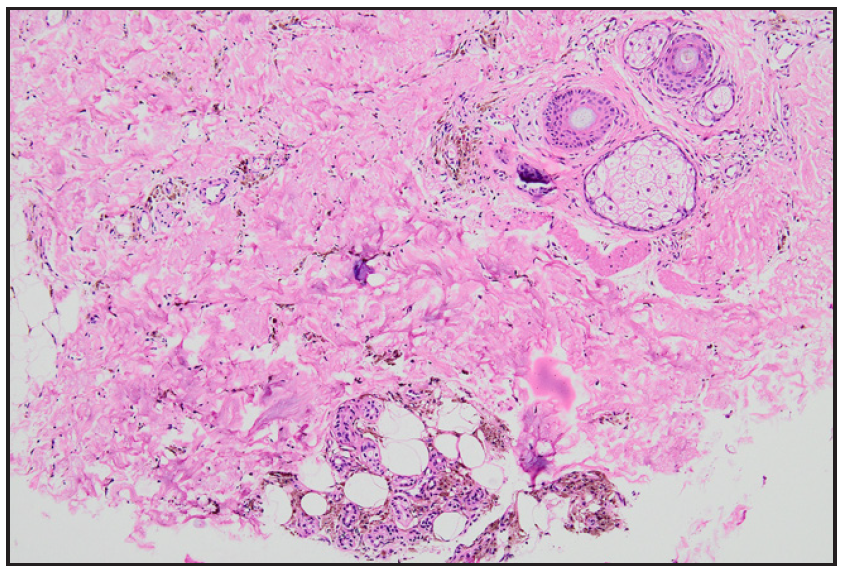
- Photomicrograph showing abundant hemosiderin around capillaries and eccrine glands in the dermis (Haematoxylin and eosin, 100x).
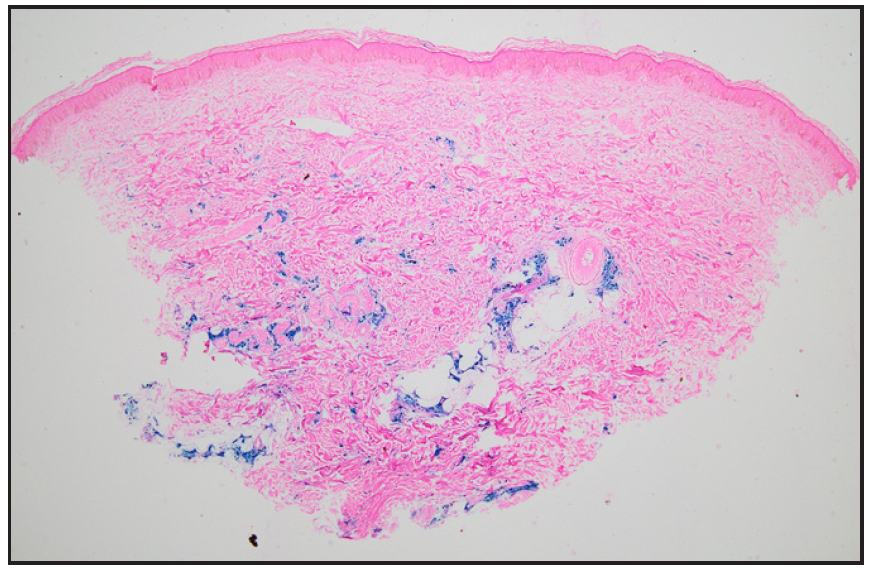
- Photomicrograph showing abundant hemosiderin around capillaries and eccrine glands in the dermis (Perl’s stain, 40x).
Despite its benefits, IV iron therapy carries a small but significant risk of skin staining due to extravasation. Skin staining occurs when iron leaks into surrounding soft tissues during infusion, resulting in persistent hyperpigmentation that can be cosmetically distressing for patients. Although rare, the incidence of iron staining has been reported in 1.6% of cases following IV iron administration.3 Iron hyperpigmentation, resembling accidental tattooing, occurs following deposition of pigment particles in dermal macrophages and subcutaneous tissue, reaching depths of up to 7 mm. Extravasation of ferric carboxymaltose or iron III saccharate commonly results in grayish-blue or brown discolouration near the infusion site. The size of iron particles vary depending on the formulation, ranging from 3 to 40 nm, which is significantly smaller than the average size of black ink particles used in tattoos (50–400 nm).4-6 The risk of extravasation and subsequent skin staining underscores the importance of adhering to strict IV administration protocols. Preventive measures include careful assessment of the infusion site, proper cannulation technique, monitoring the infusion site during the procedure, and flushing the cannula with saline before and after the infusion to minimise the risk of leakage. Moreover, patients should be adequately counselled regarding potential risk of skin staining.5-6
Cutaneous siderosis is a therapeutic challenge, and spontaneous resolution is uncommon. While several treatment approaches, such as topical agents, lymphatic drainage, and massage, have been explored with modest effectiveness, laser therapy seems to be most promising. Lasers, acting by targeting haemosiderin deposits, have demonstrated notable success in improving pigmentation, although complete resolution may require multiple sessions over an extended period. The efficacy of laser treatment is influenced by factors such as the depth of iron deposition, skin type, and the wavelength of the laser used. Both picosecond and nanosecond lasers have reported comparable outcomes for this indication . However, 24% of patients did not experience significant improvement with laser therapy, possibly due to deeper and inaccessible location of iron deposits.5,6
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Global prevalence of anemia in pregnant women: A comprehensive systematic review and meta-analysis. Matern Child Health J. 2022;26:1473-87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of iron deficiency anemia in pregnancy with intravenous versus oral iron: Systematic review and meta-analysis. Am J Perinatol. 2019;36:366-7.
- [CrossRef] [PubMed] [Google Scholar]
- Correcting iron deficiency. Aust Prescr. 2016;39:193-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of intravenous iron administration for uterine bleeding or postpartum anaemia: A narrative review. J Obstet Gynaecol. 2018;38:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of iatrogenic cutaneous siderosis with pigment lasers: A retrospective study in 15 consecutive patients. Acta Derm Venereol. 2020;100:adv00148.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of iron-induced cutaneous hyperpigmentation with energy-based devices. Lasers Surg Med. 2024;56:625-31.
- [CrossRef] [PubMed] [Google Scholar]






