Translate this page into:
Low-dose adalimumab biosimilar (ZRC-3197) in the treatment of hidradenitis suppurativa
Correspondence Address:
Sharmila Patil
OPD No. 54 First Floor, Department of Dermatology, Dr. DY Patil Hospital, Nerul East, Sector 7, Navi Mumbai - 400 706, Maharashtra
India
| How to cite this article: Patil S. Low-dose adalimumab biosimilar (ZRC-3197) in the treatment of hidradenitis suppurativa. Indian J Dermatol Venereol Leprol 2018;84:745-747 |
Sir,
Hidradenitis suppurativa is a chronic inflammatory disease mostly affecting the axillary, inguinal, and perineal areas. It clinically presents as painful, deep-seated, inflamed nodules.[1],[2] Adalimumab (Humira®), a recombinant, fully human monoclonal antibody, is the only approved TNF-α inhibitor for treatment of hidradenitis suppurativa.[2] A biosimilar adalimumab (ZRC-3197, Exemptia™; Cadila Healthcare Ltd., India), was indigenously developed as a 'fingerprint match' of Humira®, and currently approved for use in India.[3],[4] We present two cases of hidradenitis suppurativa treated with a low dose of this biosimilar adalimumab. Hidradenitis suppurativa clinical response was evaluated as percent reduction in abscess and inflammatory nodule count (AN count).
A 30-year-old overweight male (BMI 25.1 kg/m2) presented with complaints of painful discharging swellings around the buttocks, chest, beard and underarms [Figure - 1]a. He had a history of pea-sized painful swelling in the buttock that had gradually softened at the centre leading to a yellowish sticky foul smelling discharge. Multiple such lesions had appeared at different parts of his body. This restricted his shoulder mobility, indicating compromised daily activities. He was previously treated with rifampicin and doxycycline for 6 weeks, without any satisfactory outcome. Laboratory investigations, pus culture and blood pressure were normal. A diagnosis of severe hidradenitis suppurativa with Hurley's stage III disease was made. Pre-screening for infectious diseases like tuberculosis and hepatitis was negative. Treatment with biosimilar adalimumab was initiated at a low dose of 40 mg administered subcutaneously (SC) weekly for initial 3 weeks, and every other week for 3 months thereafter. Pus reduction and suppression of new lesion formation were observed after 3 months of biosimilar adalimumab therapy [Figure - 1]b. Existing lesions regressed with significant pain reduction, indicating an improved transition from Hurley stage III to I. The hidradenitis suppurativa clinical response of 50% (AN50) was achieved post 12 weeks of biosimilar adalimumab, and the reduction in severity and extent of hidradenitis suppurativa further significantly improved the quality of life. Therapy was tolerated well, and monthly maintenance dose for 6 months was continued with concomitant methotrexate to prolong the immunosuppressant activity to control inflammation and to prevent auto-antibodies against adalimumab.
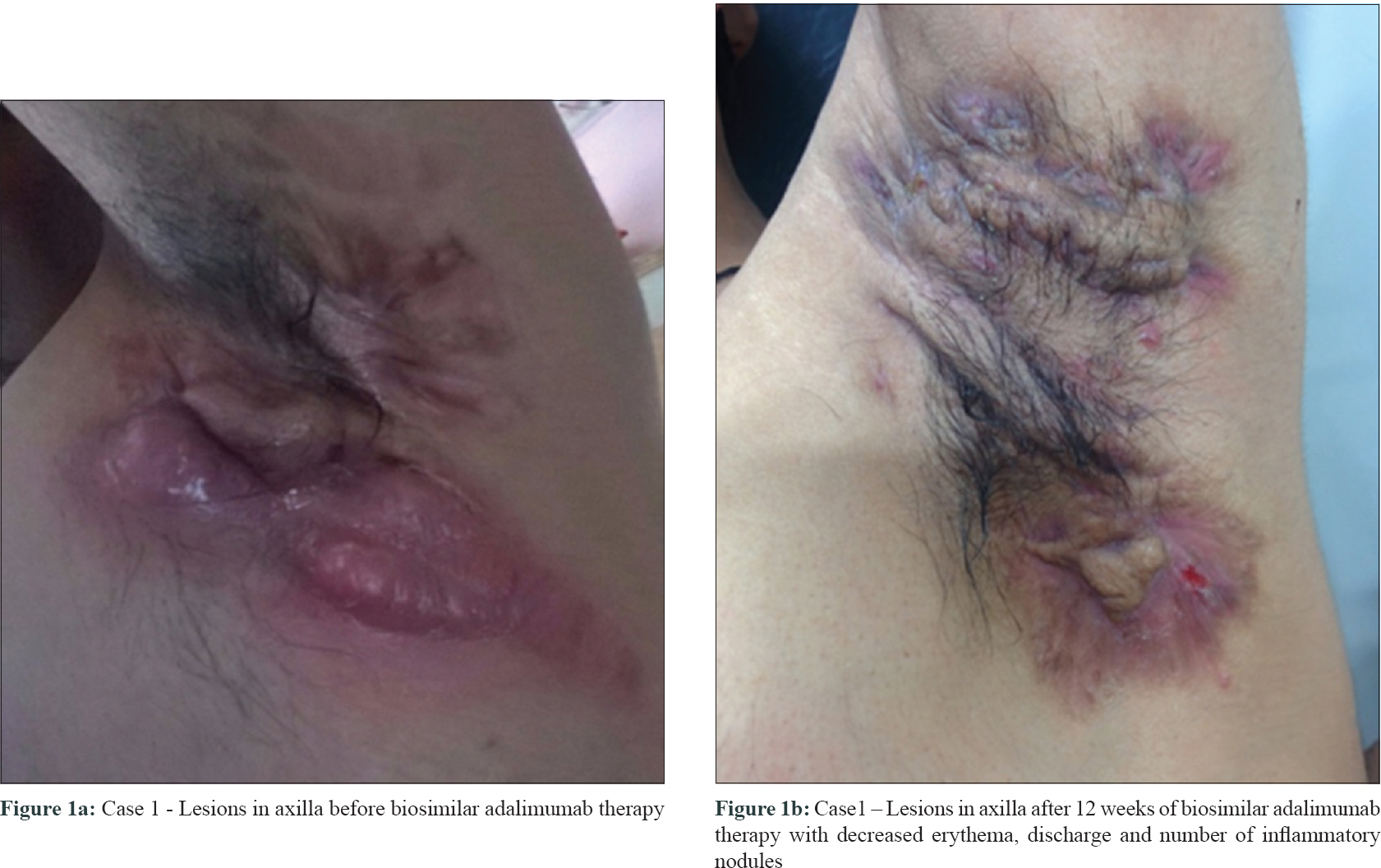 |
| Figure 1: |
Second case was a 31-year-old male (BMI 23.7 kg/m2) with a history of multiple raised painful reddish pea-sized swellings in the groins. He had undergone surgery for pilonidal sinus 12 years back. Previous treatments included rifampicin and levofloxacin for 6 weeks, and dapsone with retinoids. However, his disease progressed to form multiple nodules, abscesses, and discharging sinuses over the past 2 years [Figure - 2]a. Laboratory investigations, pus culture and blood pressure were normal. On examination, severe hidradenitis suppurativa with Hurley's stage III disease was diagnosed. Pre-screening for infectious diseases was negative. For clinically managing the recalcitrant form of hidradenitis suppurativa, a low dose of biosimilar adalimumab 40 mg SC was started weekly for 3 weeks, and thereafter every other week for 3 months. Baseline lesion count of 9 nodules and 4 sinuses reduced to 0 nodules and 2 partially healed sinuses [Figure - 2]b post biosimilar adalimumab therapy, indicating an improved transition from Hurley stage III to I. Additionally, hidradenitis suppurativa clinical response of 75% (AN75) was achieved and the patient's quality of life also improved significantly. As maintenance therapy, biosimilar adalimumab was continued monthly for 6 months with concomitant doxycycline. Additional immunosuppressants were avoided as an improvement of AN75 was obtained.
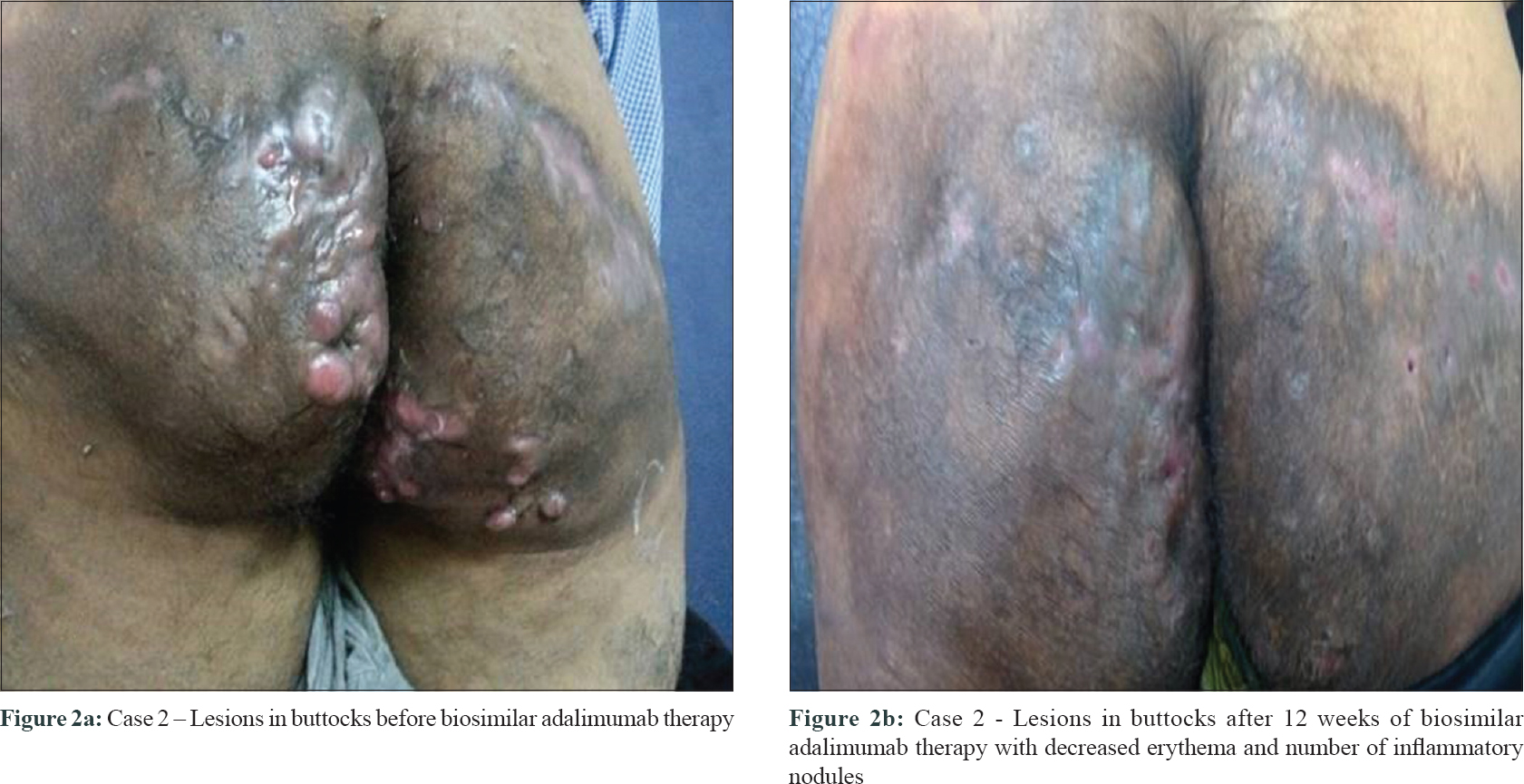 |
| Figure 2: |
Adalimumab is recommended in hidradenitis suppurativa at a dose of 160 mg SC initially, followed by 80 mg at week 2 and 40 mg weekly from week 4.[5] We considered biosimilar adalimumab at a low dose of 40 mg SC weekly for initial 3 weeks, and every other week for 3 months thereafter, as such regime allows the dosage to be modified according to the patient's response. In our cases, significant clearance of abscess and nodules prompted the dosage reduction in a tapering fashion so as to allow the disease to stay in control even with reduced dosage. While this may carry a significant amount of relapse risk post biologic discontinuation, effective management of the disease activity in a low disease activity state is easier with systemic therapy; which in turn, can help in reducing the cost of biologic therapy as well as in maintaining a significantly controlled disease activity state. If in case relapse occurs, this shall enable adalimumab to be used as a rescue agent.[6] We chose a lower than recommended dose for biosimilar adalimumab in our cases primarily due to affordability reasons, an important factor impacting treatment choices in countries like India, since cost of the therapy as per standard dosage recommendation was a big challenge. Moreover, financial factors were unlikely to have played a role in the provision of patient care. One of our patients wanted to have a disease-free time window for marriage ceremony. Hence, both cost and urgency of therapy were demanding the use of lower dose of adalimumab.
The two landmark clinical trials-PIONEER I and II-that supported the approval of adalimumab in hidradenitis suppurativa reported hidradenitis suppurativa clinical response > 50% in about 41-58% of the study patients. In our second case, the continuation of antibiotic along with biosimilar adalimumab was in concurrence with methodology adopted in PIONEER II.[7] Despite a low dose, both our patients achieved similar efficacy outcome – hidradenitis suppurativa clinical response of 50% and 75%, respectively; with an improved quality of life after biosimilar adalimumab therapy without any serious adverse drug reactions. However, we do not know how much methotrexate or doxycycline influenced the positive results. We considered a low dose biosimilar adalimumab after adequate counselling and consent from our patients, considering their financial constraints and ongoing pain. This might have reduced the chances of infections along with lesser economic burden in the short term. It is reported that there is increased chance of antidrug antibody formation at less than approved induction dosage.[8] However, in our patients, low dose biosimilar adalimumab was well-tolerated with a rapid, remarkable and sustained clinical improvement, comparable to that of the originator's adalimumab. Currently, while Indian hidradenitis suppurativa patients have no access to originator adalimumab, biosimilar adalimumab serves as a freely accessible and cost-effective treatment option.
Acknowledgments
The author would like to thank Dr. Hetal Shah, Independent Medical Writing Consultant from Ahmedabad, India for her professional support in drafting and revising this paper. Her services were funded by Cadila Healthcare Ltd., Ahmedabad, India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sibbald RG, Mufti A. Hidradenitis suppurativa CMAJ. 2015; 187:1235.
[Google Scholar]
|
| 2. |
Lee EY, Alhusayen R, Lansang P, Shear N, Yeung J. What is hidradenitis suppurativa? Can Fam Physician 2017;63:114-20.
[Google Scholar]
|
| 3. |
Bandyopadhyay S, Mahajan M, Mehta T, Singh AK, Parikh A, Gupta AK, et al. Physicochemical and functional characterization of a biosimilar adalimumab ZRC-3197. Biosimilars 2015;5:1-18.
[Google Scholar]
|
| 4. |
Jani RH, Gupta R, Bhatia G, Rathi G, Ashok Kumar P, Sharma R, et al. A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis. Int J Rheum Dis 2016;19:1157-68.
[Google Scholar]
|
| 5. |
Gupta AK, Studholme C. Adalimumab (Humira) for the treatment of hidradenitis suppurativa. Skin Therapy Lett 2016;21:1-4.
[Google Scholar]
|
| 6. |
Blanco R, Martínez-Taboada VM, Villa I, González-Vela MC, Fernández-Llaca H, Agudo M, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol 2009;145:580-4.
[Google Scholar]
|
| 7. |
Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016;375:422-34.
[Google Scholar]
|
| 8. |
Chiu HY, Wang TS, Chan CC, Lin SJ, Tsai TF. Risk factor analysis for the immunogenicity of adalimumab associated with decreased clinical response in Chinese patients with psoriasis. Acta Derm Venereol 2015;95:711-6.
[Google Scholar]
|
Fulltext Views
8,615
PDF downloads
1,246





