Translate this page into:
Low plasma zinc levels in androgenetic alopecia
2 Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Hatyai, Songkhla, Thailand
3 Department of Pathology, Faculty of Medicine, Prince of Songkla University, Hatyai, Songkhla, Thailand
Correspondence Address:
Kumpol Aiempanakit
Department of Internal Medicine, Division of Dermatology, Prince of Songkla University, Hatyai, Songkhla 90110
Thailand
| How to cite this article: Aiempanakit K, Jandee S, Chiratikarnwong K, Chuaprapaisilp T, Auepemkiate S. Low plasma zinc levels in androgenetic alopecia. Indian J Dermatol Venereol Leprol 2017;83:741 |
Sir,
Androgenetic alopecia is the most common hair loss disorder associated with androgen hormone and genetic factors. Zinc is an essential trace element and hair loss can be found in setting of zinc deficiency. Recent studies have found inconclusive results between plasma zinc levels and androgenetic alopecia;[1],[2] therefore, the authors aim to evaluate zinc levels in patients with androgenetic alopecia.
A cross-sectional case–control study was conducted in dermatology clinic of Songklanagarind Hospital, Prince of Songkla University in Southern Thailand, during 2012–through 2014. The study enrolled 114 participants, 57 patients with androgenetic alopecia (case group) and 57 age- and gender-matched without alopecia (control group). The inclusion criteria were patients with age over 18 with a history and clinical examination of androgenetic alopecia which was diagnosed by dermatologists and who had no treatment within a 3-month period before enrollment. Participants with diseases or conditions that could affect the plasma zinc levels, including nutritional deficiency, diabetes mellitus, essential hypertension, renal or liver diseases, chronic diarrhea and anemia and who were currently using nutritional supplements were excluded from the study. Data was obtained on baseline characteristics of the participants, duration and family history. A clinical pattern was determined using the Hamilton–Norwood classification in men and the Ludwig-Olsen pattern in women. Participants were subjected to a nonfasting venous blood test in the morning. The plasma zinc, albumin, blood sugar levels and white blood cell count were determined. Plasma zinc levels were measured using the Varian SpectrAA-220 atomic absorption spectrophotometry (Varian, Victoria, Australia). Albumin and blood sugar levels were measured using the Modular P800 Analyzer (Roche Diagnostics, Mannheim, Germany). White blood cell counts were measured using the Sysmex XN-3000™ (Sysmex Corporation, Kobe, Japan). Reference range for plasma zinc levels is 70–150 μg/dL and the lower cut offs for morning nonfasting plasma zinc are 66 μg/dL in females and 70 μg/dL in males.[3]
All statistical analyses were performed using Program R version 3.2.2, Epicalc version 2.15.1.0. Quantitative variables were described using mean, standard deviation, median and interquartile range. Comparisons between the two groups were conducted using chi-squared test, independent Student's t-test, and Wilcoxon signed-rank test. Univariate and multivariate analyses were conducted using linear regression analysis. P <</i> 0.05 was defined as statistically significant. The study was approved by the Research Ethics Committee in accordance with the declaration of Helsinki.
The baseline characteristics of patients with androgenetic alopecia are shown in [Table - 1]. The median age was 35 years; 30 (52.6%) participants were women and 27 (47.4%) were men. The median duration of disease was 2 years.
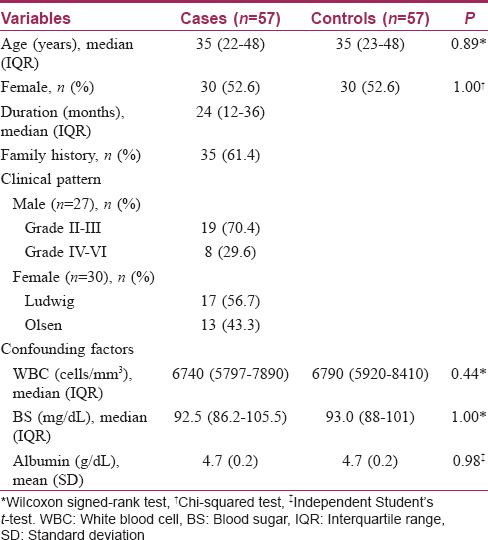
There was no statistically significant difference in participants' age, gender, or confounding factors of plasma zinc levels (white blood cell, blood sugar and albumin levels). Patients with androgenetic alopecia had mean plasma zinc levels lower than those in the control group, the difference being statistically significant (56.63 ± 11.44, 63.47 ± 11.10 μg/dL (mean ± standard deviation), respectively, P = 0.002). When analyzed by gender, both males and females in the case group had mean plasma zinc levels lower than the control group (males: 59.40 ± 12.73, 64.81 ± 10.19 μg/dL, P = 0.09; females: 54.13 ± 9.69, 62.27 ± 11.89 μg/dL [mean ± standard deviation], P = 0.005).
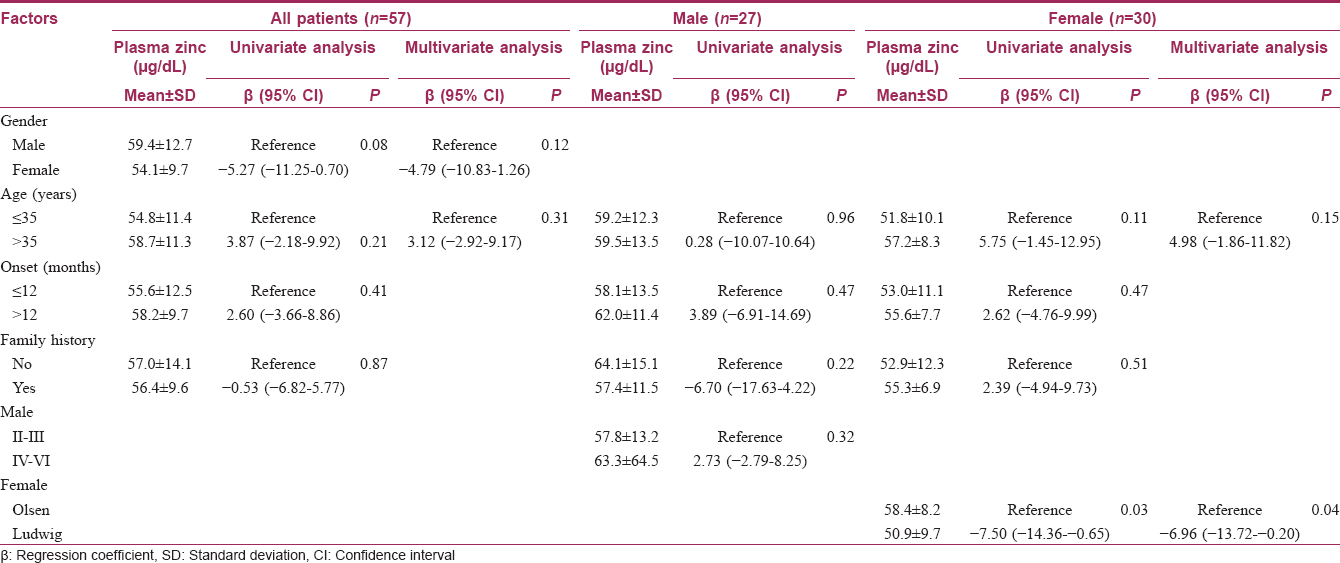
Univariate and multivariate linear regression analyses among patients with androgenetic alopecia are shown in [Table - 2]. Females with the Ludwig pattern had mean plasma zinc levels statistically significantly lower than those with the Olsen pattern.
Recent study by Kil et al. found that Koreans with androgenetic alopecia had a significant difference in serum zinc levels lower than those in the controls.[1] By contrast, Ozturk et al. did not detect a significant difference in serum zinc levels between two groups of Turkish people; however, lower zinc levels were found in the hair of the case group with statistical significance.[2] The authors hypothesized that zinc might be an essential factor in pathogenesis and may affect the treatment of androgenetic alopecia. A mouse model study showed zinc could promote regrowth of hair follicles and prevent hair loss caused by chemotherapy.[4] Nevertheless, the real effect of zinc on hair follicles is still unknown.
While a correlation between zinc homeostasis and androgenetic alopecia has been established, recent meta-analysis has associated androgenetic alopecia with metabolic syndrome.[5] Patients with metabolic syndrome have also been found to have low zinc levels.[2]
The study was limited because of the small sample size and a lack of analysis of patient's socioeconomic status and metabolic syndrome.
In conclusion, the authors found that the plasma zinc level in the participants with androgenetic alopecia was significantly lower than that found in the healthy participants. Females with the Ludwig clinical pattern had lower plasma zinc levels. A larger study should be conducted to determine whether zinc supplementation would be of benefit to androgenetic alopecia patients.
Acknowledgment
We would like to thank Dr. Kathleen Nicoletti, PhD., Faculty of Liberal Arts, Prince of Songkla University for proofreading the article and Ms. Jirawan Jayuphan, Epidemiology Unit, Faculty of Medicine, Prince of Songkla University for providing help in statistical analysis.
Financial support and sponsorship
A research fund of the Faculty of Medicine, Prince of Songkla University.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol 2013;25:405-9.
[Google Scholar]
|
| 2. |
Ozturk P, Kurutas E, Ataseven A, Dokur N, Gumusalan Y, Gorur A, et al. BMI and levels of zinc, copper in hair, serum and urine of Turkish male patients with androgenetic alopecia. J Trace Elem Med Biol 2014;28:266-70.
[Google Scholar]
|
| 3. |
Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: A review of the evidence. Br J Nutr 2008;99 Suppl 3:S14-23.
[Google Scholar]
|
| 4. |
Plonka PM, Handjiski B, Popik M, Michalczyk D, Paus R. Zinc as an ambivalent but potent modulator of murine hair growthin vivo preliminary observations. Exp Dermatol 2005;14:844-53.
[Google Scholar]
|
| 5. |
Trieu N, Eslick GD. Alopecia and its association with coronary heart disease and cardiovascular risk factors: A meta-analysis. Int J Cardiol 2014;176:687-95.
[Google Scholar]
|
Fulltext Views
4,302
PDF downloads
2,560





