Translate this page into:
Macular hypopigmentation, hair loss and follicular spongiosis: A distinct clinicopathological entity
2 Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
M Ramam
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi
India
| How to cite this article: Bhatia R, Gupta V, Arava S, Khandpur S, Ramam M. Macular hypopigmentation, hair loss and follicular spongiosis: A distinct clinicopathological entity. Indian J Dermatol Venereol Leprol 2020;86:386-391 |
Abstract
Background: Hypopigmented macules are seen in a variety of disorders and the diagnosis rests on clinicopathological correlation. However, some cases are difficult to classify and pose a diagnostic challenge.
Aim: To describe the clinical and histopathological features of patients with hypopigmented macules and follicular spongiosis on histopathology.
Materials and Methods: We undertook a retrospective analysis of clinical and histopathological findings in 12 patients who presented with clinically nondiagnostic hypopigmented macules and showed follicular spongiosis on skin biopsy, at All India Institute of Medical Sciences, New Delhi, India between January 2015 and October 2016. The findings were compared with 12 patients with “unclassified” hypopigmented macules, who did not show follicular spongiosis on skin biopsy.
Results: A total of 12 patients with hypopigmented macules showed spongiosis affecting the follicular epithelium on histopathology. There were eight men and four women, most in their second decade (mean age 19.1 ± 8.05 years), presenting with hypopigmented macules most commonly on the upper limbs, for a mean duration of 6.33 ± 5.10 months. Clinically evident lesional hair loss was seen in all patients, and follicular prominences in seven (58%) patients. Histological features suggestive of other diagnosis, namely leprosy, mycosis fungoides or sarcoidosis were not seen in any biopsy. Alcian blue stain revealed an minimal amount of mucin in one biopsy. Clinically apparent hair loss and follicular prominences were found to be statistically significantly associated with histological evidence of follicular spongiosis (P < 0.001 and 0.003, respectively).
Limitations: Our study is limited by its retrospective design and small sample size.
Conclusions: Patients with hypopigmented macules and follicular spongiosis on histopathology may represent a distinct clinicopathological entity that is associated with lesional hair loss and follicular prominences. It is probably a variant of an endogenous dermatitis similar to pityriasis alba.
Introduction
Hypopigmented macules may be seen in a wide array of diseases of varying etiology. Several of these disorders can be recognized based on their clinical and/or histopathological appearance, but a proportion of patients presenting with this finding are difficult to classify. We have noticed that some patients with macular hypopigmentation show spongiosis confined to the follicular epithelium on biopsy, in the absence of any other significant finding. We attempted to characterize this group of patients further in this study.
Materials and Methods
We evaluated the records of patients who presented to us between January 2015 and October 2016 with hypopigmented macules that were not clinically classifiable into a recognized entity and who had undergone a skin biopsy. Records of cases that showed follicular spongiosis on skin biopsy (cases) as well as those that did not show this histopathological finding (controls) were reviewed. We did not evaluate the records of patients with known causes of macular hypopigmentation such as leprosy, post-kala azar dermal leishmaniasis, sarcoidosis and progressive macular hypomelanosis, among others. Information was obtained from the records on patient details, including the duration, evolution, associated symptoms, family history, previous treatment and history of preexisting dermatoses, and clinical details of the number, size, shape, margin and sites of the lesions, erythema, scaling, atrophy, hypoesthesia and nerve thickening. Skin biopsy slides were reviewed.
Results
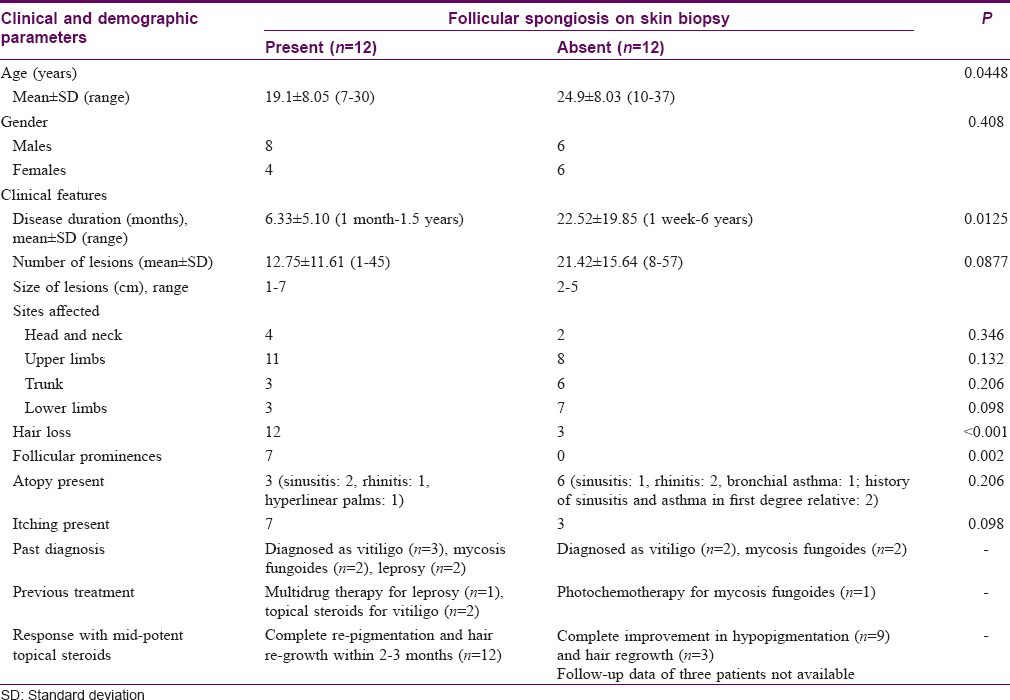
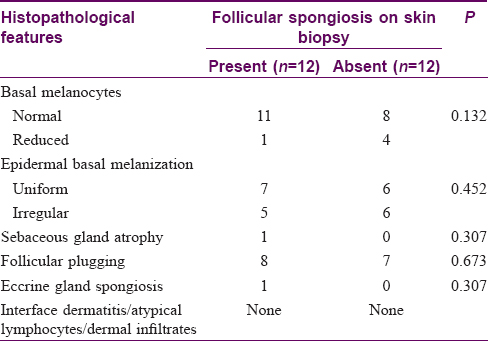
There were 24 patients who did not have the features of an identifiable disorder of hypopigmentation. In 12 of these patients, biopsy showed follicular spongiosis (cases), while in the other 12 patients, no follicular spongiosis was noted (controls). A detailed description of the cases is provided below, and their demographic, clinical and histological features are contrasted with controls in [Table - 1] and [Table - 2].
Patients showing follicular spongiosis on biopsy (cases)
There were 12 patients, aged 7–30 years (mean age 19.1 ± 8.05 years) with eight males and four females. Duration of disease ranged from 1 month to 1.5 years (mean duration 6.33 ± 5.10 months). Hypopigmented macules were present on the upper and lower limbs, trunk and neck in varying numbers. Seven patients noticed small hypopigmented macules initially that gradually increased in size over a few days and spread to other sites, while other patients could not recall how the macules evolved. Antecedent inflammatory lesions at the site of hypopigmented macules were not noted by any patient. There was mild to moderate itching in seven patients while the remaining were asymptomatic. There was no seasonal variation in disease activity. A personal or family history of atopy was present in three patients (sinusitis: 2, rhinitis: 1). History of generalized dryness was present in four cases. There was no loss of sensation over the patches or history of peripheral sensory loss in any patient. None of the patients had an illness suggestive of kala azar or sarcoidosis in the past. Two patients had received therapy for leprosy. The condition had been previously diagnosed clinically as vitiligo in three patients, mycosis fungoides in two patients and leprosy in two patients.
Cutaneous findings
Cutaneous examination revealed multiple hypopigmented macules varying in number from 1 to 45 (mean 12.8 ± 11.6) and size from 1 to 7 cm in diameter. The degree of hypopigmentation varied between macules in four patients but was similar in the remaining eight patients. The commonest sites of involvement included the upper limbs (n = 11), head and neck (n = 4), lower limbs (n = 3) and trunk (n = 3). More than one site was affected in seven patients. There was no atrophy or scaling overlying the macules. A striking feature in all the patients was a subtle but distinct hair loss over the hypopigmented macules while hair density was normal in the surrounding normally pigmented skin. Moreover, follicular prominences within the hypopigmented macules were noted in seven out of these twelve patients [Figure - 1]a, [Figure - 1]b, [Figure - 1]c, [Figure - 1]d. There was no lesional or peripheral sensory loss. There was no predilection for photo-exposed areas in any patient. Hyperlinear palms were seen in two out of three patients with history of atopy. No other features of atopy were noticed.
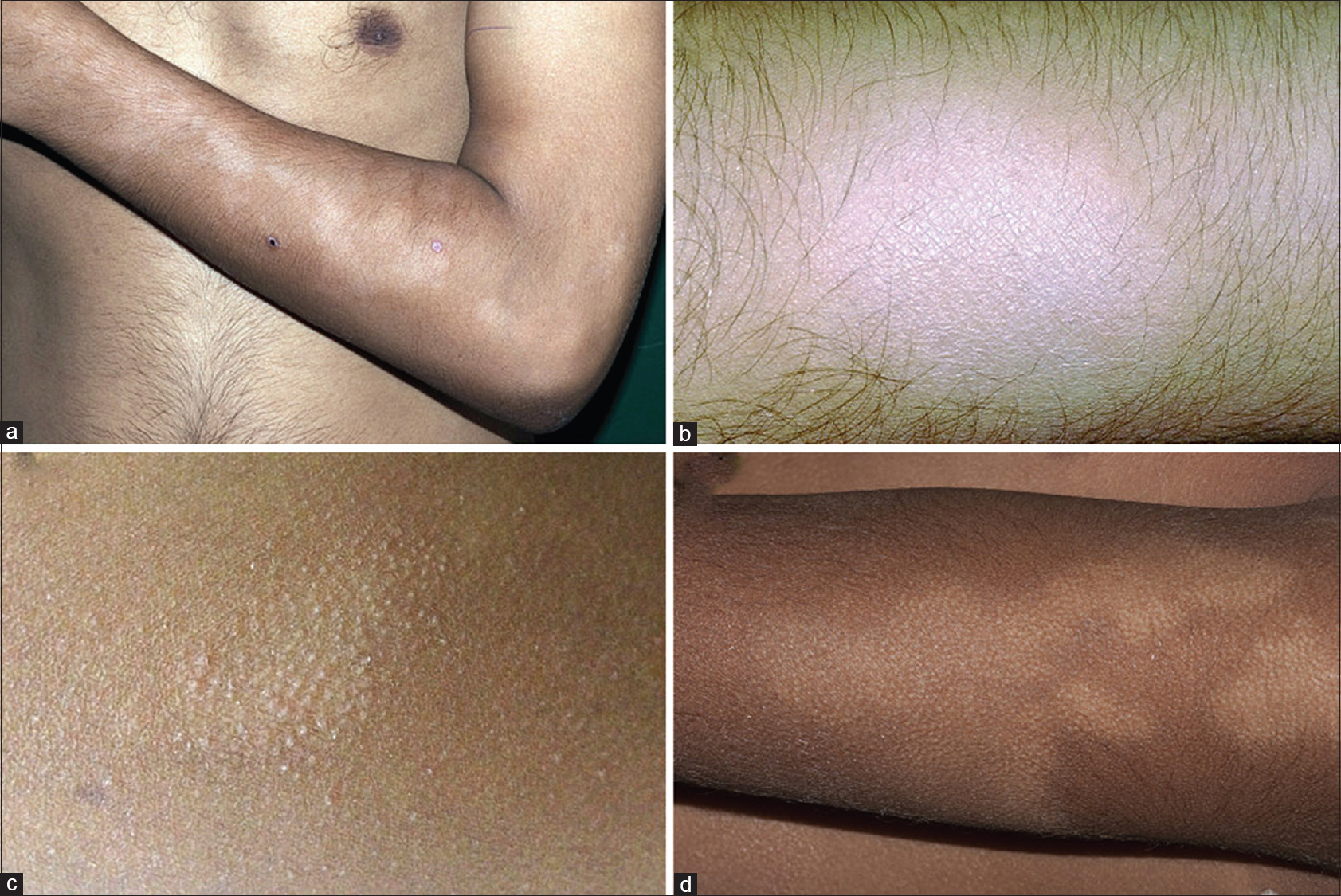 |
| Figure 1: (a) Multiple ill-defined hypopigmented macules on the forearm of a young man. (b) Loss of hair overlying the hypopigmented macule. (c) Follicular prominences overlying the hypopigmented macule. (d) Hair loss as well as follicular prominences overlying the hypopigmented macule |
Histopathological analysis
There was prominent follicular spongiosis in all the patients (n = 12), accompanied by lymphocyte exocytosis limited to the follicular epithelium [Figure - 2]a, [Figure - 2]b, [Figure - 2]c, [Figure - 2]d, [Figure - 2]e, [Figure - 2]f. In six patients, multiple sections were required to identify these findings. Follicular spongiosis was graded as mild (n = 2), moderate (n = 8) and severe (n = 2) according to the degree of spongiosis and the intensity of lymphocyte exocytosis. In three out of 12 cases, there was focal spongiosis in the epidermis immediately adjacent to the involved follicle. Perifollicular inflammation varied in intensity: it was mild in seven cases, moderate in three and marked in two cases. There was no evidence of follicular destruction, perifollicular fibrosis or basal layer vacuolation in epidermis or follicles. Other findings included follicular plugging (n = 8), parakeratotic follicular plug overlying the follicle showing spongiosis (n = 2) and spongiosis of the eccrine duct (n = 1). Sebaceous glands could be identified in six biopsies, of which one showed atrophy while the others were normal. No follicular or dermal mucin could be identified in any biopsy on hematoxylin and eosin stain. Alcian blue stain revealed a small amount of mucin in the follicular epithelium in one biopsy, while it was absent in the others. The number of melanocytes were evaluated both on sections stained with hematoxylin and eosin and the immunohistochemical stain for melanocytes (Melan-A). The number of basal melanocytes was normal in 11 cases and reduced in one case.
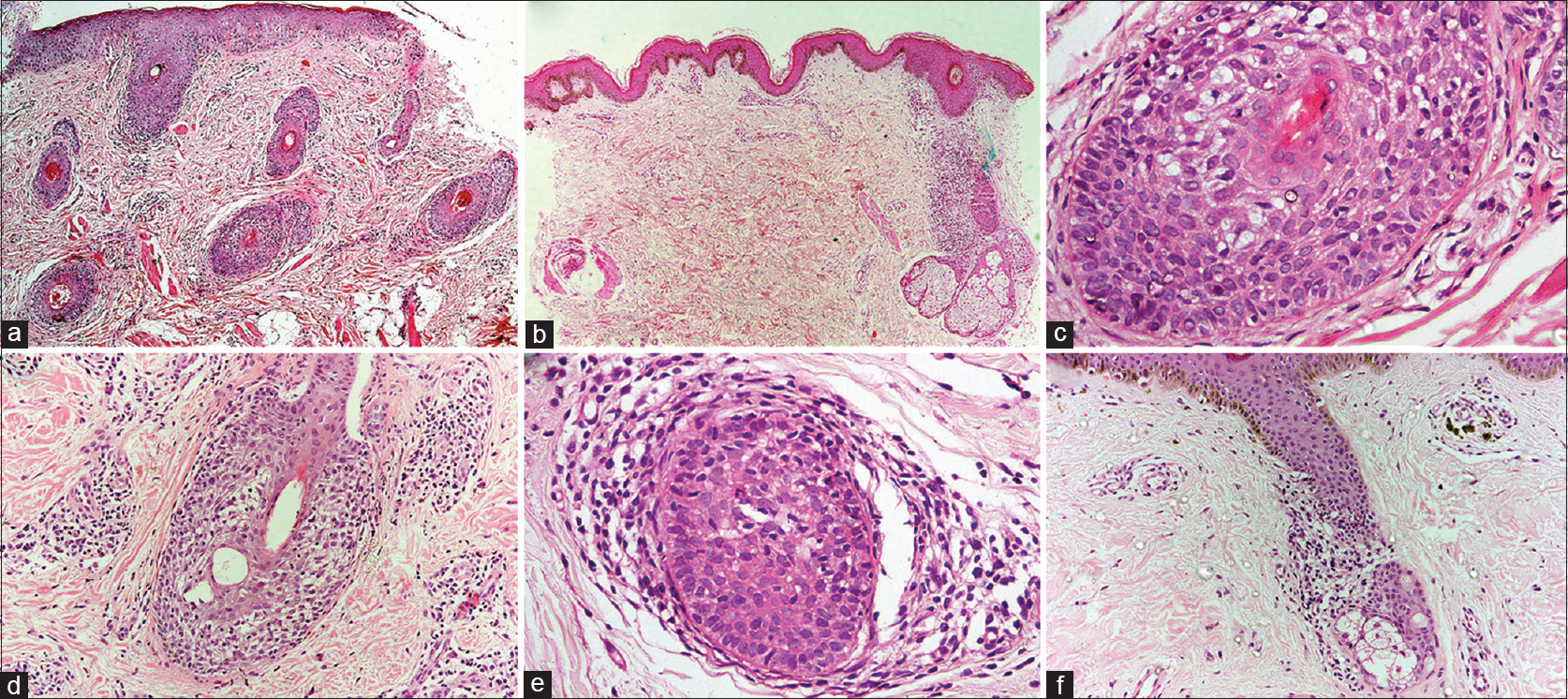 |
| Figure 2: (a) Multiple hair follicles in the dermis show spongiosis. No spongiosis is seen in the overlying epidermis (hematoxylin and eosin, ×100). (b) Perifollicular infiltrate of lympho-histiocytes along with follicular spongiosis. There is spongiosis affecting the epidermis overlying the hair follicle as well(hematoxylin and eosin, ×100). (c) Spongiosis of the follicular epithelium with lymphocytic exocytosis (hematoxylin and eosin, ×400). (d) Spongiosis of the follicular epithelium with lymphocytic exocytosis (hematoxylin and eosin, ×400). (e) Spongiosis of the follicular epithelium with lymphocytic exocytosis (hematoxylin and eosin, ×400). (f) Spongiosis of the follicular epithelium with lymphocytic exocytosis (hematoxylin and eosin, ×200) |
Epidermal basal layer melanization was uniform in seven cases and irregular in the rest. None of the biopsies showed any interface change, enlarged, haloed or atypical lymphocytes in the epidermis or dermis, or upper dermal infiltrates.
Treatment response
All patients were initially treated with emollients with no change in 2 months. Moderately potent topical corticosteroids were then prescribed. Patients were then followed up at intervals of 2–3 months and complete improvement was noticed in hypopigmentation and hair regrowth in all patients within 1–2 months of initiation of treatment in all patients.
Discussion
Several dermatological conditions present with hypopigmented macules as the predominant or exclusive finding. These include indeterminate leprosy, pityriasis alba, hypopigmented mycosis fungoides, hypopigmented sarcoidosis, early vitiligo, post kala-azar dermal leishmaniasis, progressive macular hypomelanosis, lichen sclerosus, leukoderma syphiliticum and postinflammatory hypopigmentation, among others.[1] Many of these conditions can be recognized clinically, while others require histopathology and/or ancillary investigations for diagnosis. However, some patients cannot be categorized into any of these known entities. We studied a subset of such patients that showed a recognizable clinicopathological pattern.
These patients had hypopigmented macules that were clinically non-descript except for lesional hair loss and follicular prominence. The significant histological finding in these cases was spongiosis of the follicular epithelium and lymphocytic exocytosis. Though some of these clinical and histological features can be seen in other disorders, the combination of these clinicopathological features seems to be unique to this entity.
Hypopigmentation accompanied by hair loss is one of several clinical presentations of tuberculoid leprosy. However, it is usually accompanied by sensory loss, nerve thickening or sensory/motor neuropathy that were absent in our patients. Its histopathology is characterized by superficial and deep, perivascular and periappendageal, oblong and curvilinear, epitheloid cell granulomas. Follicular spongiosis is not a feature.[2]
Hypopigmented mycosis fungoides presents with hypopigmented macules but hair loss is not a feature. The often intense epidermotropism and haloed lymphocytes typical of this condition were not seen in our cases. Though we did not perform clonality studies because of lack of facilities, the test is of limited utility in diagnosing or excluding early mycosis fungoides in the absence of suggestive clinical or histopathological findings.[3] Folliculotropic mycosis fungoides may show hair loss in about 65% of patients but it presents with patches and plaques that are usually erythematous or hyperpigmented and are indistinguishable from classic mycosis fungoides.
In post kala-azar dermal leishmaniasis, biopsy from hypopigmented macules shows a sparse inflammatory infiltrate, mainly consisting of lymphocytes, histiocytes and some plasma cells, aggregated around the vessels and hair follicles in the superficial dermis.[2] Hypopigmented sarcoidosis was ruled out in view of lack of sarcoidal granulomas.[4] Progressive macular hypomelanosis which is characterized by asymptomatic ill-defined macules over trunk, with a tendency to coalesce around the midline has a non-descript histological finding of reduced basal layer melanization.[5]
As a histopathological finding, follicular spongiosis has been described previously in follicular atopic eczema, disseminated infundibulo-folliculitis, seborrheic dermatitis, Fox-Fordyce disease and follicular mucinosis.[6],[7],[8] However, none of these diseases manifest clinically as hypopigmented macules. Follicular atopic eczema is characterized by discrete or grouped, itchy papules predominantly on the trunk of atopic children. Disseminated infundibulo-folliculitis presents as asymptomatic or mildly itchy, small, skin-colored, smooth, discrete, follicular papules on the trunk. Seborrheic dermatitis has erythematous plaques with greasy scales affecting the seborrheic sites. Fox-Fordyce disease is characterized by extremely itchy, skin-colored, small papules in areas rich in apocrine glands. Hair loss can be seen in follicular mucinosis, but it is an erythematous boggy swelling affecting the head and neck region. In addition, there is mucin deposition in the follicular epithelium which was not seen in any of our cases. Perifollicular inflammation was mild in most of our cases. Perifolliculitis is a feature of disseminate and recurrent infundibulo-folliculitis, infective folliculitis and various scarring and nonscarring primary follicular inflammatory disorders. However, considering the clinical picture of hypopigmented macules with follicular papules and hair loss along with predominant follicular spongiosis on histopathology, none of these is a close differential of our cases.
Vargas-Ocampo's description of 39 cases of pityriasis alba has a close clinical and histopathological resemblance to our series.[9] Follicular spongiosis was seen in 30 of 39 biopsies in their series. In contrast to our findings, Vargas-Ocampo noted follicular spongiosis in erythematous and hypopigmented patches with follicular papules and in only one out of five patients with smooth, hypopigmented macules. However, we wish to emphasize the presentation of macular hypopigmentation with follicular spongiosis, often accompanied by hair loss as it poses particular diagnostic problems in our patients. This presentation was not mentioned by Vargas-Ocampo and is not generally recognized as a manifestation of extra-facial pityriasis alba.[9] Follicular spongiosis was not described in a more recent study of 56 patients of pityriasis alba.[10]
Although our cases bear some resemblance to pityriasis alba, there are differences. Classically, pityriasis alba is a disease of preadolescents affecting the face more than extremities and trunk and the macules are ill-defined and occasionally itchy with a winter exacerbation. Our cases had begun in adulthood with well-defined hypopigmented macules with discernible hair loss, predominantly on extremities without any seasonal predilection. Hair loss appears to be due to primary follicular damage rather than a response to scratching since it occurred more frequently in non-itchy patients. Follicular spongiosis and exocytosis represent injury to the hair follicle which probably led to hair loss by unknown mechanisms.
Limitations
Our study is limited by its retrospective design and small sample size and more detailed studies with a prospective design and a larger sample size are required to confirm our findings.
Conclusions
The combination of clinical and histopathological findings noted in our cases may represent a distinct entity. Because of several similarities, the designation “truncal pityriasis alba” may be suitable for this condition or the more descriptive but non-committal “hypopigmenting dermatitis” may be used till the etiopathogenesis and nosology of the condition is better worked out. Many of these patients are given a diagnosis of leprosy or mycosis fungoides in our part of the world. Knowledge of this entity will help avoid misdiagnosis and inappropriate treatment.
Acknowledgement
We are grateful to Dr. CP Yadav, Department of Biostatistics, All India Institute of Medical Sciences for his help in statistical analysis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Patel AB, Kubba R, Kubba A. Clinicopathological correlation of acquired hypopigmentary disorders. Indian J Dermatol Venereol Leprol 2013;79:376-82.
[Google Scholar]
|
| 2. |
Singh A, Ramesh V. Histopathological features in leprosy, post-kala-azar dermal leishmaniasis, and cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol 2013;79:360-6.
[Google Scholar]
|
| 3. |
Pimpinelli N, Olsen EA, Santucci M, Vonderheid E, Haeffner AC, Stevens S, et al. Defining early mycosis fungoides. J Am Acad Dermatol 2005;53:1053-63.
[Google Scholar]
|
| 4. |
Clayton R, Breathnach A, Martin B, Feiwel M. Hypopigmented sarcoidosis in the Negro. Report of eight cases with ultrastructural observations. Br J Dermatol 1977;96:119-25.
[Google Scholar]
|
| 5. |
Relyveld GN, Menke HE, Westerhof W. Progressive macular hypomelanosis: An overview. Am J Clin Dermatol 2007;8:13-9.
[Google Scholar]
|
| 6. |
Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol 1979;115:174-5.
[Google Scholar]
|
| 7. |
Ackerman AB, Boer A, Bennin B, Gottlieb GJ, editors. Histological Diagnosis of Inflammatory Skin Diseases, a Method by Pattern Analysis. 1st ed. Philadelphia: Lea and Feibiger; 1978.
[Google Scholar]
|
| 8. |
Cockerell C, Mihm MC Jr., Hall B, Chisholm C, Jessup C, Merola MC. Dermatopathology. London: Springer-Verlag; 2014.
[Google Scholar]
|
| 9. |
Vargas-Ocampo F. Pityriasis alba: A histologic study. Int J Dermatol 1993;32:870-3.
[Google Scholar]
|
| 10. |
In SI, Yi SW, Kang HY, Lee ES, Sohn S, Kim YC. Clinical and histopathological characteristics of pityriasis alba. Clin Exp Dermatol 2009;34:591-7.
[Google Scholar]
|
Fulltext Views
8,437
PDF downloads
2,432





