Translate this page into:
Management of pain in the inpatient and non-surgical outpatient dermatology settings: A narrative review
Corresponding author: Dr. Keshavamurthy Vinay, Department of Dermatology, Venereology and Leprology, PostGraduate Institute of Medical Education and Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bishnoi A, Shah S, Jain S, Reddy A, Singh V, Lad D, et al. Management of pain in the inpatient and non-surgical outpatient dermatology settings: A narrative review. Indian J Dermatol Venereol Leprol. 2024;90:742-9. doi: 10.25259/IJDVL_331_2023
Abstract
Pain is frequently encountered in dermatology practice, which impairs the activities of daily living, adds to psychological morbidity, and therefore compromises the quality of life. It ranges from mild to severe in intensity across various dermatoses and requires prompt addressal and treatment. Diseases such as extensive pemphigus vulgaris and Stevens–Johnson syndrome are especially painful and require a multidisciplinary approach with the involvement of a pain specialist in their management. The main pathogenic types of pain include visceral nociceptive, somatic nociceptive, and neuropathic types, the latter two being most relevant in dermatological disorders. Somatic nociceptive pain is often seen in patients of Stevens–Johnson syndrome/ Toxic epidermal necrolysis, epidermolysis bullosa, pemphigus vulgaris, erythema nodosum, and hidradenitis suppurativa, while neuropathic pain is part of the disease process in dermatoses like leprosy, herpes zoster, and dysesthesia syndromes. Therapeutic approaches to pain management include the use of non-opioids (acetaminophen, non-steroidal anti-inflammatory agents), opioids, and non-pharmacological therapies, along with appropriate management of the underlying dermatosis. World Health Organisation (WHO) analgesic ladder remains the most commonly employed guideline for the management of pain, although treatment needs individualisation depending on the nature and severity of pain (acute/chronic), type of dermatosis, and patient factors.
There is a paucity of literature pertaining to pain management in dermatology and this topic is often neglected due to a lack of awareness and knowledge of the topic. The present review aims to discuss the pain pathway, various painful conditions in the setting of medical dermatology practice, and their management along with relevant pharmacology of the commonly used analgesics.
Keywords
pain
opioids
pain in dermatology
Introduction
Pain is defined by the International Association for the Study of Pain as – an unpleasant sensory and emotional experience associated with, or resembling that associated with actual or potential tissue damage.1
There are different classifications of pain based on duration, pathophysiology, and aetiology [Table 1].2 Acute pain is usually associated with an underlying insult, whereas chronic pain lasts beyond the normal healing time which is often 3–6 months after the insult.3 The worldwide prevalence of chronic pain is around 30–40% in various surveys.4-6 A study in India found the prevalence of chronic pain to be around 19.3%.7 The main pathogenic types include visceral nociceptive, somatic nociceptive, and neuropathic, the latter two being most relevant to dermatological disorders.
| Somatic nociceptive | Visceral nociceptive | Neuropathic | |
|---|---|---|---|
| Site |
Superficial: skin and mucosa Deep: bone, joints, muscles |
Viscera: internal abdominal organs | Injury to nerve cells in the peripheral or central nervous system |
| Localisation | Well localised | Poorly localised | Poorly localised |
| Character |
Superficial: sharp or burning Deep: dull or aching |
Diffuse and dull aching or cramping | Needles, tingling, burning, sharp, or shooting |
| Examples |
Superficial: Oral lichen planus especially erosive variants, pemphigus, SJS-TEN, EM, pyoderma gangrenosum Deep: necrotising soft tissue infection, sclerosing disorders with contractures, EB, GVHD |
Myocardial infarction | Postherpetic neuralgia, pyoderma gangrenosum, leprosy, scleromexedema |
SJS-TEN: Steven Johnson Syndrome-Toxic Epidermolysis Necrosis; EM: Erythema Multiforme; EB: Epidermolysis Bullosa; GVHD: Graft vs Host disease
Pain is a common complaint among dermatology patients.8 While chronic itch and chronic pain share similar pathways, there are differences in predominant cytokines involved. Itch is mediated by IL-2, IL-13, and IL-31, while pain is mediated by IL-1β, IL-6, CCL2, CCL5, and CXCL1.9 All three opioid receptor subtypes, mu, delta, and kappa, are known to mediate analgesia both centrally and peripherally. Itch is triggered only by µ opioid receptor agonists, delta agonists having no effect, and kappa agonists being able to attenuate itch.
While mild pain can be controlled with over-the-counter medications, certain conditions may warrant the involvement of a pain specialist. Pain limits the daily activities of a patient, adds to the psychological morbidity, and deteriorates the quality of life.2 Despite this pressing need for the management of pain by dermatologists, pertinent training is often lacking.3
The most commonly employed guideline for the management of pain is the World Health Organisation (WHO) analgesic ladder [Figure 1].4,10 Originally developed for cancer pain, its spectrum has widened. For acute pain, the strongest analgesic needed to control pain is the initial therapy, and stepping down is done later according to the requirement and response of the patient (top to bottom). For chronic pain, a step-wise approach from bottom to top is usually employed. The three main principles of the WHO analgesic ladder are: “By the clock, by the mouth, by the ladder” which means that analgesia should be provided at all times of the day and night, preferably by oral route following the step ladder approach.
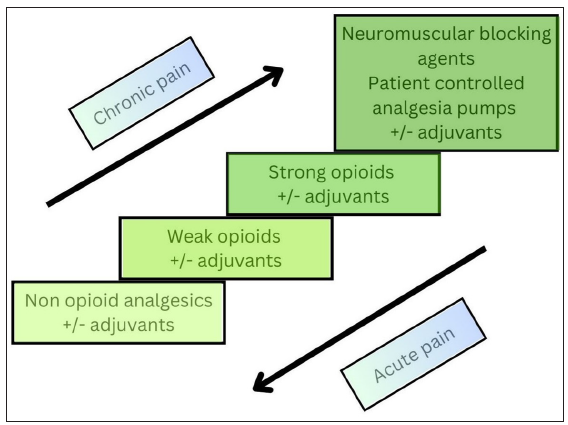
- Modified World Health Organization (WHO) analgesic ladder.
Pain pathway
Analgesia is defined as loss of sensation of pain without associated loss of consciousness. The pain pathway comprises three major steps which are as follows:
Cutaneous transduction via free nerve endings
Processing at the level of the spinal cord
Projection to the higher neuronal centres
The pain pathway is depicted in a schematic diagram in Figure 2, various medications alleviate pain by acting on step(s) of this pathway. 11,12
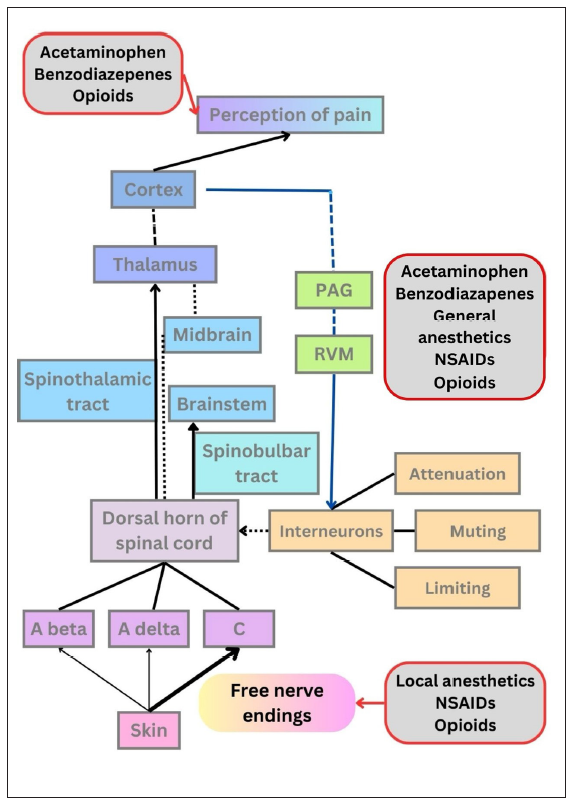
- Schematic representation of the pain pathway. The first step is cutaneous transduction in which the free nerve endings (nociceptors) act as sensory receptors and the impulse reaches the primary sensory afferent fibres (A-beta, A-delta, and C). The second step is the processing of the impulse at the spinal cord where interneurons modulate the pain by limitation, muting, and attenuation. This is explained by the gate theory of neurotransmission. In the third step, pain signals travel via neuronal projection systems (spinothalamic and spinobulbar pathways) to higher centers like the brainstem, thalamus, and cortex where the pain is perceived. Descending pathways (depicted by blue lines) that pass receive inputs from periaqueductal grey (PAG) and rostral ventromedial medulla (RVM) have modulatory action on interneurons. NSAIDS: Non-steroidal anti-inflammatory drugs.
Painful dermatologic conditions with predominantly superficial/ somatic nociceptive pain
Pemphigus vulgaris (PV), toxic epidermal necrolysis (TEN), epidermolysis bullosa (EB), and hidradenitis suppurativa (HS) are some of the common dermatologic conditions associated with severe and acute somatic nociceptive pain. Due to mucosal erosions, administration of analgesics via parenteral routes may be preferred, especially in the acute phase. Skin fragility precludes the usage of transdermal analgesics, and sometimes even placement of IV lines, and other routes like nasal sprays might need to be employed in those settings.
While the bottom to top pain ladder can be followed in some dermatoses associated with chronic somatic nociceptive pain (e.g., erythema nodosum, HS); for others (e.g., TEN, PV, generalised fixed drug eruption), pain is usually acute and severe enough (visual analogue scale (VAS)≥6) to warrant administration of strong analgesics like opioids from the beginning (top to bottom). Additionally, the culprit drug is often unknown in TEN, so it is advisable to avoid nonsteroidal anti-inflammatory drugs (NSAIDs). VAS scoring can be repeated roughly at 4 hourly intervals for severely painful conditions like Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), where a score of <=4 can be considered to be optimal pain control while >4 may warrant use the of stronger analgesics/opioids or adjustment of dosage of the drugs.
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN)
Pain assessment using VAS and optimisation of pain control is important in SJS/TEN. The international multidisciplinary Delphi-based consensus can be followed for pain and psychological stress management. [Table 2].5
| 1. The severity of pain and the efficacy of pain medications should be assessed at regular intervals |
| 2. Evaluation and treatment of pain should be a priority in the acute-phase management of SJS/TEN, particularly during wound care |
| 3. The efficacy of pain medications should be assessed with a visual analogue scale according to the age of the patient |
| 4. Opioids should be used in most cases of SJS/TEN, NSAIDs are usually avoided |
| 5. High-potency opioids (e.g. morphine) should be used if the visual analogue scale score is elevated |
| 6. Non-oral formulations of opioids (e.g. intranasal diamorphine or sublingual fentanyl) may be used for limited procedures unless active disease in these distributions precludes its local use |
| 7. Non-opioid agents (e.g. ketamine infusions) may be used over opioids during wound care in the intensive care unit |
| 8. Sedation and mechanical ventilation may be used to achieve pain control |
| 9. Psychiatric and/or psychological evaluation should be affected to reduce post-traumatic stress disorder |
SJS-TEN: Steven Johnson Syndrome-Toxic Epidermolysis Necrosis; NSAIDS: Non-steroidal anti-inflammatory drugs
Due to the severity of pain and ease of monitoring in hospital settings, non-oral opioids are preferred, especially before/during dressing and/or position changes. High-potency opioids (e.g., morphine 0.15–0.2 mg/kg) should be used. The absorption from oral, intramuscular, and subcutaneous routes can be erratic therefore intravenous route is preferred. Another advantage of the intravenous route is patient-controlled analgesia (PCA). Non-opioid agents (e.g., ketamine infusions 0.1–0.5 mg/kg) may be preferred over opioids during wound care in the intensive care unit.
Once shifted to home care, other non-oral opioids (e.g., intranasal diamorphine spray, 0.1 mg/kg; intranasal [1.5 mcg/kg] or sublingual fentanyl tablet [100 mcg]) may be used for limited procedures and dressing changes. Additionally, alprazolam can be used to prevent anxiety and resultant pain from the dressing change.
Epidermolysis Bullosa (EB)
Pain in EB can be nociceptive or neuropathic and acute or chronic.6 Poorly managed pain can adversely impact the development of the nervous system of the child and result in psychological comorbidities.7 Guidelines proposed by the European Reference Network for Rare Skin Diseases include:8
Clinical assessment of pain in EB
-
-
For 2 months–7 years of age: Faces, Legs, Activity, Cry, Consolability scale (FLACC scale)9
-
-
7 years onwards: Visual analogue scale (VAS), Numeric rating scale, Faces pain scale
-
-
Neuropathic pain screening tool – Douleur Neuropathique – A score of more than 4 out of 10 is suggestive of neuropathic pain with a sensitivity of 78–83% and specificity of 81–90%.13 It has not been validated for children and adolescents.
Therapeutic Strategies for pain management in EB
Management of nociceptive or neuropathic pain [Table 3]
Premedication for dressing changes
| Indication | Medication |
|---|---|
| Mild pain (NRS or FLACC <4/10) | Non opioid analgesics (acetaminophen, ibuprofen) |
| Moderate pain | Weak opioid analgesic (Nefopam, tramadol, codeine) |
| Severe pain | Strong opioid, Fentanyl, Morphine |
| Minor procedures | Topical anaesthetics |
| Chronic pain | Tricyclic antidepressants or anti-epileptics |
NRS, Numeric Rating Scale; FLACC, Face, Leg, Activity, Cry, Consolability Scale
The non-pharmacologic approach includes a calm and soothing atmosphere, comfortable ambient temperature, and making sure the patient is well fed. For chronic pain management, cognitive behavioural therapy can be added to the regimen.
When intravenous (especially true in long-standing scarring seen in dystrophic EB) and enteral routes are unavailable, transmucosal (including intranasal fentanyl 1–2 mcg/kg and trans-buccal fentanyl formulations 2–4 mcg/kg) should be considered for short procedures. Pre-procedural use of benzodiazepines (e.g., alprazolam 5 mcg/kg) offers the benefit of anterograde amnesia which may prevent the development of increased anxiety with repeated procedures. When using benzodiazepines in combination with opioids, care must be taken to avoid over-sedation.14 It is of utmost importance to wait for adequate duration for the drugs to act before starting the dressing removal and bathing process (oral acetaminophen [30–45 minutes], tramadol [30–45 minutes], morphine [15–30 minutes], oxycodone [15–30 minutes], intranasal fentanyl [15–20 minutes], intravenous fentanyl [5–10 minutes]). Topical morphine mixed in hydrogel formulations has been used in localised painful wounds but varying absorption spectrum and safety profile require further investigations before recommendation. Studies are evaluating the topical application of diluted injection formula of ropivacaine 0.2% on EB wounds and oral administration of injectable ketamine (1–2 mg/kg).15 Other analgesic agents include acetaminophen and NSAIDS (when postoperative bleeding is not a risk and renal function is normal; e.g., ketorolac). Patient-controlled analgesia (PCA) pump technology is a safe and efficient way to deliver pain relief by allowing the patient to administer a predetermined bolus dose of medication at the press of a button along with a background infusion of medication. However, PCA is not preferred in children less than 7 years old.
Other genodermatoses like Ehler-Danlo Syndrome (EDS) and hereditary palmoplantar keratodermas (PPK) may also have pain as a clinical feature. Early stages of EDS are associated with nociceptive pain due to damage to joints, managed with anti-inflammatory drugs and opioids.16 In the late stages, neuropathic pain predominates, managed with antidepressants and anticonvulsants. Physiotherapy remains crucial. Pain is a prominent symptom in some PPKs such as pachyonychia congenita and Olmsted syndrome.17 Treatment includes managing the underlying disease and the addition of gabapentin, with anecdotal evidence for botulinum toxin injection.
Pemphigus vulgaris
Pain is a major complaint in pemphigus patients and significantly contributes to morbidity.18 Despite this, there are no randomised controlled trials or standard regimen described. Management relies on controlling disease activity and inducing remission.19 Pain control is essentially similar to TEN and opioid therapy fentanyl/morphine is the cornerstone analgesia approach to achieve a pain VAS score ≤4/10 till remission is achieved. In severe cases, especially during a wound dressing change, deep sedation or even general anaesthesia may be warranted to prevent psychological distress. Standard non-adherent wound dressings (e.g. silver dressings, petroleum jelly gauze) or biosynthetic dressings coupled with adequate wetting during removal have been shown to be beneficial in the control of pain.20 In resource-poor settings, more cost-effective alternatives for dressing such as autoclaved banana leaves and potato peels have been utilised in pain control and inducing re-epithelialisation.20,21 Dexmedetomidine (0.25–0.5 mcg/kg/min infusion) has also been tried and found to be an effective periprocedural adjuvant. Gabapentin (300 mg once daily at night to three times a day with the maximal dose being 3600/day) is also used to relieve neuropathic pain. Newer therapies such as CO2 laser, azathioprine mouth rinses, and topical tacrolimus ointment are currently under evaluation for pain control in pemphigus.21
For the management of mucosal erosions except eyes, gel formulations of topical benzocaine provide adequate analgesia.22 It should be applied 30 minutes before eating or drinking. It is uncommonly associated with minor adverse effects including local burning or irritation.
Genital mucosal pain can be distressing. Apart from vulvodynia and penodynia; erosive dermatoses, infections, and lichen sclerosus might be predominantly painful.23 Attempts should be made to identify and manage the underlying dermatosis.
Erythema nodosum
Symptomatic treatment is important for rapid relief of the patient’s distress.24 Compression bandage and elevation of the limb are non-pharmacological measures. Pharmacologic therapy mainly involves NSAIDs such as indomethacin (50–70 mg thrice a day, maximum up to 200 mg/day) and naproxen (350–375 mg twice a day) but should be used carefully in patients having underlying inflammatory bowel disease since they may trigger a flare-up.
Hidradenitis Suppurativa
Addressing pain is an important aspect of HS.. A treatment algorithm was recently proposed by Savage et al.25 It includes the identification of pain as acute or chronic, which is further classified as nociceptive or neuropathic if chronic. Acute pain is usually managed with acetaminophen and/or topical NSAIDs, especially if mild, in association with anti-inflammatory antibiotics and surgical drainage. If uncontrolled, oral NSAIDs and/or intralesional triamcinolone can be added. If still persistent, a short course of opioids such as tramadol can be utilised but the patient should be referred to a pain specialist.
Chronic pain is usually secondary to disease activity per se and thus management of the disease, and combining surgical intervention usually helps in alleviating the pain. Nociceptive pain can be managed with NSAIDs or celecoxib (COX-2 inhibitor), and if severe, second-line options include duloxetine (Serotonin norepinephrine reuptake inhibitor – SNRI) or nortriptyline (Tricyclic antidepressant – (TCA). For neuropathic pain, gabapentin and duloxetine (30 mg PO up to 60 mg/day) are the first-line treatment options while pregabalin (50 mg PO TID up to 300 mg/day), venlafaxine (SNRI) (37.5 mg PO daily up to 225 mg/day) and nortriptyline (25 mg PO QHS up to 150mg/day) are the second-line agents. Adjunctive therapies like topical NSAIDs and topical lidocaine can be used in all patients. If persistent and/or severe, the patient should be promptly referred to a pain specialist. A similar line of pain management can be extrapolated to acne conglobata, acne, and rosacea fulminans.
Painful dermatologic conditions with predominantly neuropathic pain
Dermatologic conditions with predominantly neuropathic pain comprise mainly leprosy, herpes zoster and post-herpetic neuralgia (PHN), dysesthesia syndromes such as vulvodynia, notalgia, and meralgia paresthetica, burning mouth syndrome, trigeminal trophic syndrome and squamous cell carcinoma with perineural invasion among many others. Additionally, a component of neuropathic pain is also present in many chronic diseases.
PHN is a common debilitating complication of herpes zoster. The prevention and treatment strategies are summarised in Table 4.26,27
| Prevention | Treatment |
|---|---|
| The most important is advocating vaccination for high-risk groups which has an efficacy of preventing herpes zoster up to 89–91% with Shingrix. | Conventional Jaipur block comprises of a combination of xylocaine, bupivacaine, and dexamethasone which is injected at the site of maximum pain. |
| Early initiation of anti-viral therapy | Modified Jaipur block utilises methylprednisolone instead of dexamethasone for a better anti-inflammatory action - Intralesional steroids combat the inflammatory response and increase the perineural penetration of anaesthetics. |
| Optimisation of symptomatic pain therapy | |
| Early sympathetic blocks | |
| Oral corticosteroid – limited evidence |
In leprosy, pain is a frequent complaint that adds to the morbidity and may persist after treatment completion.28 The management of pain involves early and effective Multidrug Therapy and anti-inflammatory therapy. Steroids are an effective means to control the pain of lepra reactions.
Chronic neuropathic pain is usually managed by combining oral medications [Table 5] and topical analgesics and anaesthetics along with physiotherapy.28,29 Topical lidocaine is available in the form of 5% cream, 5% patch, 4% ophthalmic drops, etc. It can rarely cause local irritation and pain. Capsaicin acts by the counter irritant mechanism and is available as 0.025%, 0.075% and 0.1% creams in combination with other counterirritants, and also as 8% patch formulation. It is avoided on open skin for the risk of severe burning and pain.
| Class name | Drugs | Usual adverse effects |
|---|---|---|
| Anticonvulsants |
Gabapentin: Starting dose: 100–300 mg thrice/day Maximum dose: 1200 mg per day Pregabalin: Starting dose: 50–100 mg thrice per day Maximum dose: 300 mg per day |
Dizziness, irritability, drowsiness, memory problems, weight gain, dry mouth, fatigue, and peripheral oedema. |
| Tricyclic antidepressants |
Amitriptyline, Nortriptyline and Desipramine Starting dose: 25 mg/day Maximum: 150 mg/day |
Weight gain, dry mouth, blurring of vision, drowsiness, arrythmias, and orthostatic hypotension, may potentiate the anticholinergic action of antihistamines. |
| Serotonin norepinephrine reuptake inhibitors (SNRI) |
Duloxetine Starting dose: 30 mg/day Maximum: 120 mg/day Venlafaxine Starting dose: 37.5 mg/day Maximum: 225 mg/day |
Nausea, dizziness, weight loss, constipation, decreased libido, insomnia. Not as effective as TCA for neuropathic pain but has a better side effect profile |
TCA, Tricyclic Antidepressants
Dosing and recommendations for common analgesics
Acetaminophen
Acetaminophen is a well-tolerated drug but it is not a suitable substitute for NSAIDs in chronic inflammatory conditions.28 An acute overdose can result in hepatic failure which makes it relatively contraindicated in patients with concomitant liver disease and alcohol abuse. It is present in many combination agents thus care must be taken to avoid inadvertent overdose.30
Non-steroidal anti-inflammatory drugs
Unless contraindicated, management of all levels of pain includes an NSAID.28 Although they belong to the same class, there can be differences in response to various agents, and thus a therapeutic trial of 1–2 weeks is recommended.31 The side effects are primarily dose-dependent. Chronic therapy with NSAIDs warrants lab monitoring with complete blood count, blood urea nitrogen, creatinine, and liver enzymes at least once yearly.30 More frequent monitoring is required in patients with renal compromise, liver disease, pre-existing anaemia, and in cases of concomitant use with diuretics, angiotensin converting enzyme (ACE) inhibitors, and cyclosporine.
Side effects include gastrointestinal adverse effects – dyspepsia, peptic ulcer disease; renal side effects – decreased glomerular filtration rate, hyperkalemia, hypersensitivity reactions, Increased risk of cardiovascular events, severe drug reactions including SJS/TEN.
Opioids
Opioids are a controlled substance group of analgesics and are usually reserve drugs (bottom to top) for severely painful conditions and/or if other therapies fail. They have a substantial risk of addiction and potential for adverse cardiorespiratory events which precludes their widespread usage. However, certain acute dermatological conditions usually warrant first-line albeit short-duration of opioids with minimal risk of addiction (top to bottom).
Treatment is individualised and is usually started at a low dose titrated upwards to find the lowest effective dose. Periodic re-evaluation for pain, assessment of tolerance, the addition of adjunctive therapies, and tapering of opioid dose should be done. Treatment is usually initiated with shorter-acting opioids every 4–6 h until adequate analgesia is achieved.32 Once the pain is under control, either low-dose short-acting agents are continued or switched to longer-acting opioids. Eventually, two-thirds of the daily requirement are met by long-acting formulation, with doses of an immediate-acting drug taken on an as-needed basis (10–15% of total opioid dose).33
For example, if the pain in a patient is controlled during the initial 2 days with morphine sulfate immediate release 10 mg every 4 h, making the total dose per day as 60 mg; after 2 days, this could be converted to morphine sulfate sustained release 30 mg twice a day. Reassessment of pain should be done daily. For breakthrough pain, for example during a dressing change, one- sixth of the total dose, that is 10 mg of immediate-release morphine sulfate can be administered as and when needed.
In the presence of erosions, these drugs may be given parenterally using a syringe driver that delivers the drug subcutaneously over 24 h. The dose of the drug for parenteral administration is usually half of the oral dose.
It is important to be aware of conversion dosages and pharmacokinetics of various opioids when switching from one to another. For example, oxycodone and fentanyl are better options than morphine in case of mild-moderate and severe renal dysfunction, respectively. Fentanyl and buprenorphine are both available in transdermal and formulations. A few simple conversions are: The analgesic potency of tramadol and codeine is 10% of that of parenteral morphine and oxycodone is 1.5 times more potent than morphine; 12.5 micrograms/ hour fentanyl patch is equivalent to 30 mg oral morphine over 24 hours and 10 microgram/ hour buprenorphine patch is equivalent to 24 mg oral morphine over 24 hours.
US FDA defines a patient as opioid-tolerant if they require oral morphine 60 mg/day, transdermal fentanyl 25 mcg/hour, oral oxycodone 30 mg/day, or an equianalgesic dose of another opioid for at least a week. In case of tolerance, increase the current opioid dose (10–20%), switch to a different opioid, and/or add a non-opioid to the current regimen.34 The indications of different drugs in the class of opioids vary based on their duration of action and additional properties [Table 6].32 The side effect profile of opioids include:35 sedation, nausea, cognitive impairment, urinary retention, respiratory depression, pruritus, sexual dysfunction, and constipation.
| Generic name | Formulations and routes | Dosing schedule | Bioavailability | Duration of action (hrs) | Additional features |
|---|---|---|---|---|---|
| Codeine | Oral | 15–60 mg q4h | 4% | 3–4 | 10–25% as potent as morphine. Used for mild to moderate pain |
| Oxycodone | Oral | 5–10 mg q4-6h | 87% | 3–4 | Sustained release available, usually prescribed 12 hourly. Available with acetaminophen or aspirin. Used for moderate to severe pain |
| Morphine | Oral | 10–30 mg q4-6h | 30% | 3–5 | Sustained release available, dosed 15–30 mg twice or thrice daily. Used for moderate to severe pain |
| Intramuscular | 10–20 mg q4h | 100% | 4–5 | ||
| Intravenous | 0.1–0.2 mg/kg q4h | 100% | 4–5 | ||
|
Hydro- morphine |
Oral | 2–4 mg q4-6h | 10–65% | 4–5 | Shorter acting than morphine, 6–7 times more potent. Used for severe pain |
| Subcutaneous | 1–2 mg q2-3h | 78% | 3–4 | ||
| Intramuscular | 1–2 mg q2-3h | 92% | 3–4 | ||
| Intravenous | 0.2–1 mg q2-3h | 100% | 3–4 | ||
| Methadone | Oral | 2.5–10 mg q6-8h | 70% | 6–8 | Long half-life, dose escalation after at least 3 days. Used for moderate to severe pain |
| Fentanyl | Lozenge | 200–1600 mcg | 50% | 2–3 | 75–100 times more potent than morphine. Used for severe pain. |
| Effervescent tablet | 100–800 mcg | 65% | 3–4 | ||
| Soluble film | 200–1200 mcg | 71% | 2–6 | ||
| Sublingual tablet | 100–800 mcg | 54% | 7–8 | ||
| Sublingual spray | 100–1600 mcg per spray | 76% | 1–1.5 | ||
| Nasal spray | 50–200 mcg per spray | 89% | 1 | ||
| Tramadol | Oral | 50–300 mg q12-24h | 75% | 4–6 | Also a serotonin and norepinephrine reuptake inhibitor, less respiratory depression. Used for mild to moderate pain |
| Intramuscular | 50–100 mg 4–6 h | 100% | 5–6 | ||
| Intravenous | 50–100 mg 4–6 h | 100% | 5–6 | ||
| Acetaminophen | Oral | 325–650 mg q4-6h or 1gm q8h | 79% | 4–6 | Avoid doses >3000 mg for the risk of hepatotoxicity. Used for non-inflammatory pain |
| Intravenous | 650 mg q4h or 1g q6h | 100% | 5–6 | ||
| Diclofenac | Oral | 50 mg q8-12h | 50–60% | 8 | |
| Transdermal (1–2%) | NA | 6.6% | 2–3 | ||
| Suppository | 50–100 q12h | 90% | 15 | ||
| Intramuscular | 50–100 mg q12-24h | 100% | 6–7 | ||
| Intravenous | 37.5 mg q6h | 100% | 6–7 | ||
| Indomethacin | Oral | 25–50 mg q8-12h | 100% | 5–10 | Especially useful in inflammatory pain in the joints |
| Suppository | 25–50 mg q8-12h | 80–90% | 5–10 | ||
| Piroxicam | Oral | 10–20 mg q24h | 90% | 50 | Preferably prescribed by pain specialists. Avoid taking both piroxicam and aspirin. |
| Ibuprofen | Oral | 400 mg q4-6h | 100% | 6–8 | Additional anti-inflammatory potential. Gastrointestinal side effects prominent |
| Intravenous | 400–800 mg q6h | 100% | 6–8 | ||
| Naproxen | Oral | 250–500 mg q12h | 100% | 12–17 | Especially useful in inflammatory pain in the joints |
| Aspirin | Oral | 325–1000 mg q4-6h | 50% | 240 | Anti-platelet action in low doses |
| Celecoxib | Oral | 200 mg q24h | 22–40% | 11 | Selective NSAID |
NSAID, Nonsteroidal anti-inflammatory drug; NA, Not applicable
Non-pharmacologic complementary therapies
Nonpharmacologic techniques like vibration, cold anaesthesia, verbal distraction, music, hypnosis, guided imagery, communication, and patient education can be employed as adjuvants for pain management.36
Carboxytherapy is the subcutaneous injection of CO2 a technique mainly used in aesthetic dermatology but its purview has been extended to the management of post-operative neuropathic facial pain, peripheral neuropathy, and fibromyalgia.37
Pain management during pregnancy and lactation
Pain medications taken in prescribed dosage are generally safe during pregnancy.38 The lowest effective dose should be used. Due to the potential for antiplatelet effects, women should refrain from using NSAIDs beyond 32 weeks of pregnancy.39 Additionally, opioids should be taken cautiously in late pregnancy due to potential risks to the newborn like poor foetal growth, stillbirth, and preterm labour, and the neonate should be closely monitored for any signs of withdrawal (neonatal abstinence syndrome).
Conclusion
To conclude, pain is a common complaint among dermatology patients and is often neglected partly due to a lack of adequate awareness regarding pain management. Appropriate pain management in dermatological conditions can improve the quality of life of the patients and lead to faster clinical recovery. Pain management should therefore be a part of standard teaching in dermatology residency programs.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- The revised international association for the study of pain definition of pain: Concepts, challenges, and compromises. Pain. 2020;161:1976-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The impact of pain on quality of life and the unmet needs of pain management: Results from pain sufferers and physicians participating in an Internet survey. Am J Ther. 2008;15:312-20.
- [CrossRef] [PubMed] [Google Scholar]
- Gaps in pain management in dermatology: A needs assessment from Canada. J Am Acad Dermatol. 2014;71:1258-9.
- [CrossRef] [PubMed] [Google Scholar]
- WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7:93-6.
- [PubMed] [Google Scholar]
- Supportive care in the acute phase of Stevens-Johnson syndrome and toxic epidermal necrolysis: An international, multidisciplinary Delphi-based consensus. Br J Dermatol. 2021;185:616-26.
- [CrossRef] [PubMed] [Google Scholar]
- Pain quality assessment scale for epidermolysis bullosa. Acta Derm Venereol. 2018;98:346-49.
- [CrossRef] [PubMed] [Google Scholar]
- Procedural sedation and analgesia in pediatric patients. J Pediatr Neurosci. 2014;9:1-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Practical management of epidermolysis bullosa: Consensus clinical position statement from the European reference network for rare skin diseases. J Eur Acad Dermatol Venereol. 2021;35:2349-60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical validation of FLACC: Preverbal patient pain scale. Pediatr Nurs. 2003;29:140-6.
- [PubMed] [Google Scholar]
- WHO Analgesic Ladder. 2023 Apr 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Cellular and molecular mechanisms of pain. Cell. 2009;139:267-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pain Theory. 2023 Apr 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018;17:456-66.
- [CrossRef] [PubMed] [Google Scholar]
- Dystrophic Epidermolysis Bullosa Research Association International (DEBRA International). Pain care for patients with epidermolysis bullosa: Best care practice guidelines. BMC Med. 2014;12:1-23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of topical ropivacaine in children and young adults with hereditary epidermolysis bullosa. Br J Dermatol. 2021;184:550-2.
- [CrossRef] [PubMed] [Google Scholar]
- Pain in ehlers-danlos syndrome: A non-diagnostic disabling symptom? Healthcare (Basel). 2023;11:936.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pain mechanisms in hereditary palmoplantar keratodermas. Br J Dermatol. 2020;182:543-51.
- [CrossRef] [PubMed] [Google Scholar]
- Pain management in patients with severe pemphigus vulgaris. J Pain Palliat Care Pharmacother. 2022;35:278-82.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus pain: A review on management. Clin J Pain. 2008;24:734-5.
- [CrossRef] [PubMed] [Google Scholar]
- Banana leaves as a traditional dressing for pemphigus: A case report. Iran J Dermatol. 2022;25:66-9.
- [CrossRef] [Google Scholar]
- Novel pain management therapies for patients with pemphigus. Int J Dermatol 2022 Epub ahead of print
- [CrossRef] [PubMed] [Google Scholar]
- Topical anesthetics in dermatology. J Am Acad Dermatol. 2000;43:286-98.
- [CrossRef] [PubMed] [Google Scholar]
- Erythema nodosum: A practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-99.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevention of Post-herpetic Neuralgia from dream to reality: A ten-step model. Pain Physician. 2017;20:E209-E220.
- [CrossRef] [PubMed] [Google Scholar]
- Post herpetic neuralgia: A retrospective study to evaluate response to modified jaipur block with increased concentration of dexamethasone. Indian J Dermatol. 2021;66:459-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of pain associated with selected conditions in dermatology. Am J Clin Dermatol. 2016;17:463-74.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic pain management in dermatology: A guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-73.
- [CrossRef] [PubMed] [Google Scholar]
- Pain: pathophysiology and management. In: Fauci A, Longo DL, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill; 2012.
- [Google Scholar]
- The appropriate use of non-steroidal anti-inflammatory drugs in rheumatic disease: Opinions of a multidisciplinary European expert panel. Ann Rheumat Dis. 2011;70:818-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic pain management in dermatology: Pharmacotherapy and therapeutic monitoring with opioid analgesia. J Am Acad Dermatol. 2015;73:575-82. quiz 583–4
- [CrossRef] [PubMed] [Google Scholar]
- Acute pain management in dermatology: Risk assessment and treatment. J Am Acad Dermat. 2015;73:543-60.
- [CrossRef] [PubMed] [Google Scholar]
- Pain management with opioids in 2019-2020. JAMA. 2019;322:1912-3.
- [CrossRef] [PubMed] [Google Scholar]
- A review of non-pharmacologic approaches to enhance the patient experience in dermatologic surgery. Dermatol Online J. 2020;26
- [PubMed] [Google Scholar]
- Analgesic action of invasive carboxytherapy: Mechanisms and applications. Neurophysiology. 2021;53:56-64.
- [CrossRef] [Google Scholar]
- Treating pain during pregnancy. Can Fam Physician. 2010;56:25-7.
- [PubMed] [PubMed Central] [Google Scholar]
- BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part II: analgesics and other drugs used in rheumatology practice. Rheumatology (Oxford). 2016;55:1698-702.
- [CrossRef] [PubMed] [Google Scholar]






