Translate this page into:
Massive retiform hemangioendothelioma that expresses D2-40
Corresponding Author:
Xia Lei
Department of Dermatology, Daping Hospital, The Third Military Medical University, Chongqing 400042
China
leixia1979@sina.com
| How to cite this article: Tan Y, Hu Y, Wu J, Cheng Q, Lei X. Massive retiform hemangioendothelioma that expresses D2-40. Indian J Dermatol Venereol Leprol 2017;83:360-363 |
Sir,
A 42-year-old woman was admitted to Daping Hospital, The Third Military Medical University, Chongqing, China. She had a history of erythema, papules, nodules, and plaques on her left leg for more than 30 years. Two years prior to this, the skin lesions enlarged, became coalescent and spread to the left hip and calf. Severe muscle atrophy was observed in the left thigh and hip, along with mild tenderness [Figure 1a] and [Figure 1b]. In addition, a large, diffuse dark-red plaque with clear margins, was observed on the left hip and left thigh. Her vital signs were stable. Some papules/nodules were located on the periphery and some were interspersed within the plaque. These lesions were plump and had scaly, rough surfaces. When the lesions were palpated, warmth, mild tenderness, a moderately spongy consistency and low skin elasticity were detected. Isolated papules and nodules, approximately 1–3 cm in diameter, were observed on the left calf and some were ruptured or scabbed. Lymphography of the legs indicated a lymphatic obstruction and infiltrates in the middle left thigh [Figure - 2]. The pathological characteristics of the biopsy specimen were as follows: a retiform pattern (similar to rete testis tissue) was observed in the dermis and subcutaneous tissues. The vascular channels were lined by monomorphic endothelial cells with apical nuclei and sparse cytoplasm which presented a hobnail pattern and protruded into the lumen. A large number of lymphocytes and spindled or epithelioid cells were observed around the lumen. Regional intravascular papillae with collagenous cores were observed [Figure 3a] and [Figure 3b]. Immunohistochemically, endothelial staining was positive for D2-40, CD34, CD31 and Ki-67 (the positive labeling index of Ki-67 was 15%) [Figure 3c],[Figure 3d],[Figure 3e],[Figure 3f]. Smooth muscle actin staining was observed in the vessel wall, but was negative in endothelial cells [Figure 3g]. A microscopic examination of the surgically resected sentinel lymph node showed no evidence of metastasis. According to the above findings, the patient was diagnosed with retiform hemangioendothelioma.
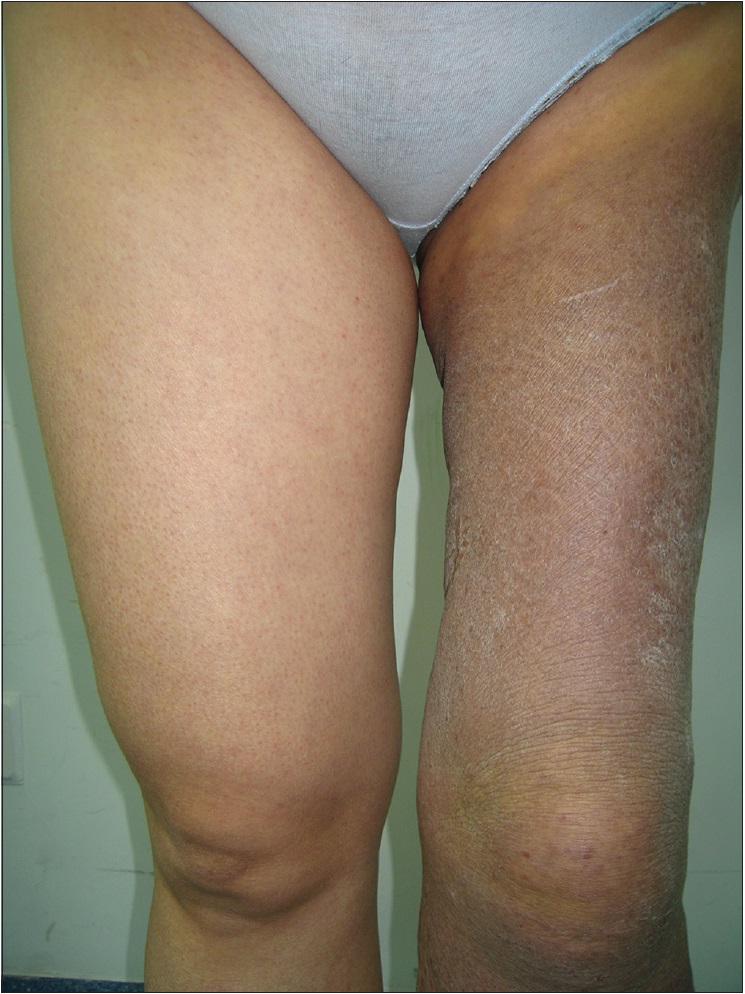 |
| Figure 1a: Severe muscle atrophy was observed in the left thigh and hip and a large, diffuse distribution of dark-red large plaques with clear margins were observed on the left hip and left thigh. Isolated papules and nodules, approximately 1-3 cm in diameter, were observed on the left calf (front view) |
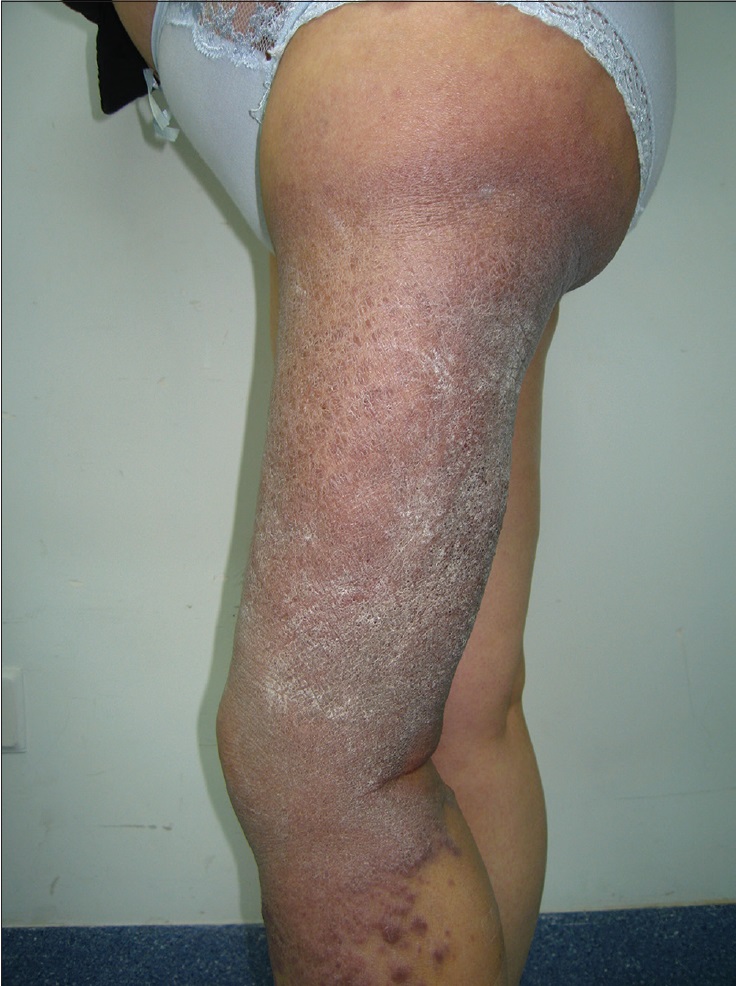 |
| Figure 1b: Isolated papules and nodules, approximately 1-3 cm in diameter, on the left calf (lateral view) |
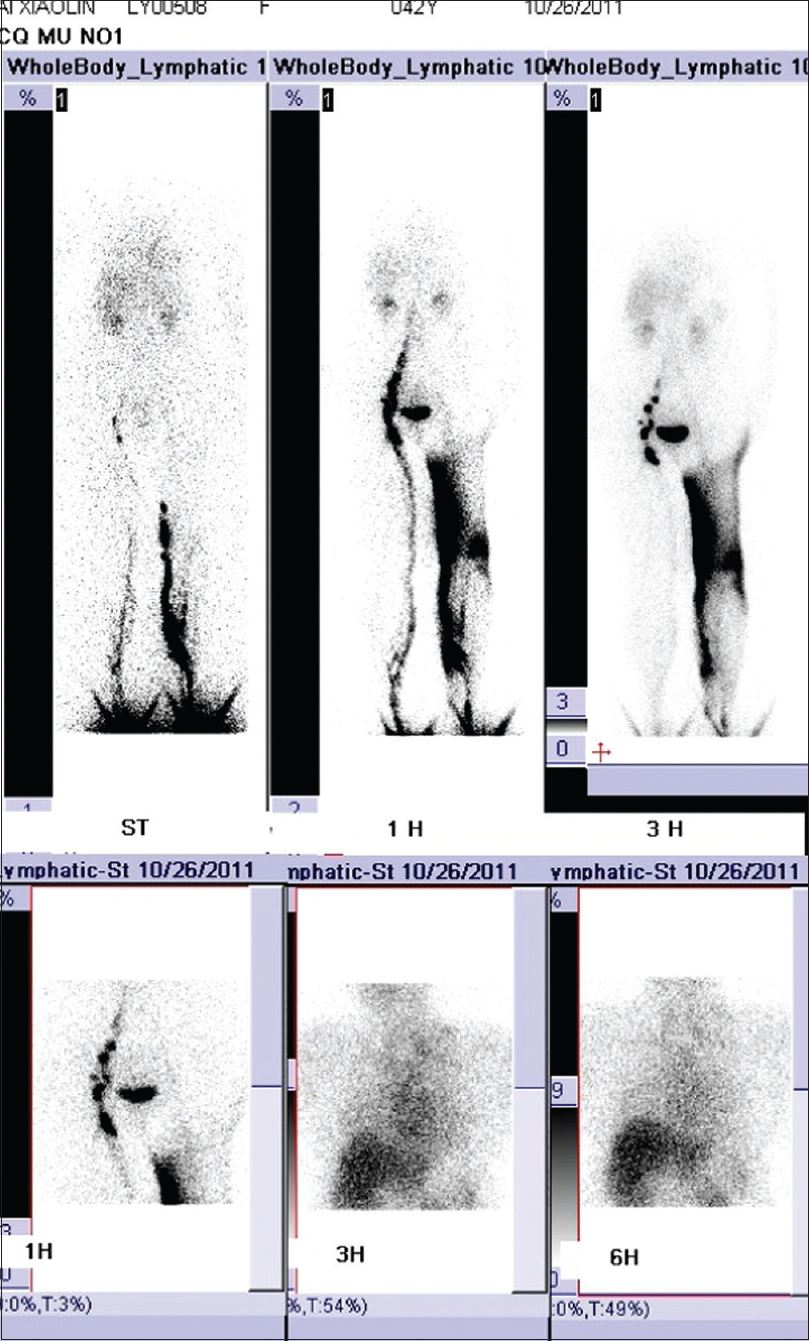 |
| Figure 2: Lymphography of the legs indicated a lymphatic obstruction and infiltrates in the middle left thigh |
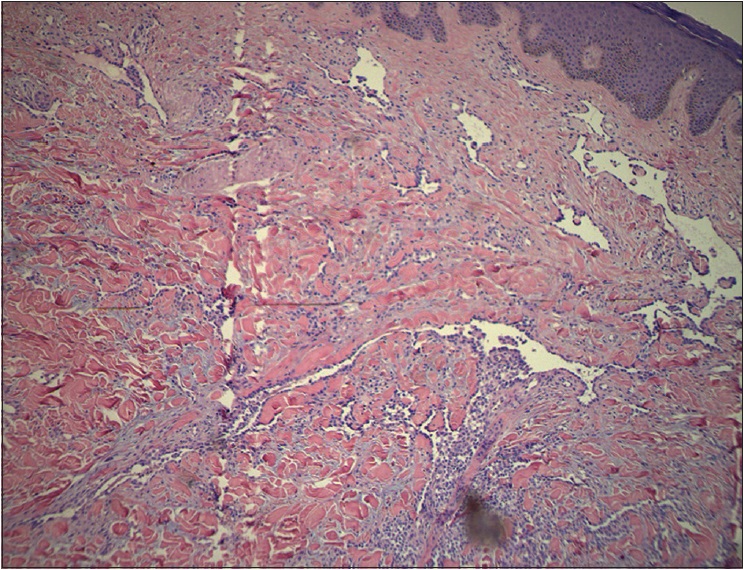 |
| Figure 3a: A retiform pattern was observed in the dermis and subcutaneous tissues. The vascular channels were lined by monomorphic endothelial cells with a hobnail pattern. Focally, intravascular papillae with collagenous cores were observed (H and E, ×100) |
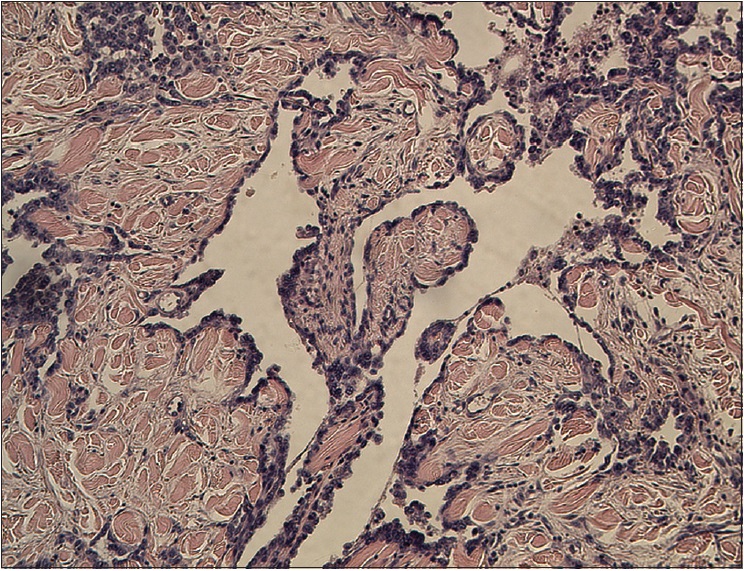 |
| Figure 3b: A retiform pattern was observed in the dermis and subcutaneous tissues. The vascular channels were lined by monomorphic endothelial cells with a hobnail pattern. Focally, intravascular papillae with collagenous cores were observed (H and E, ×200) |
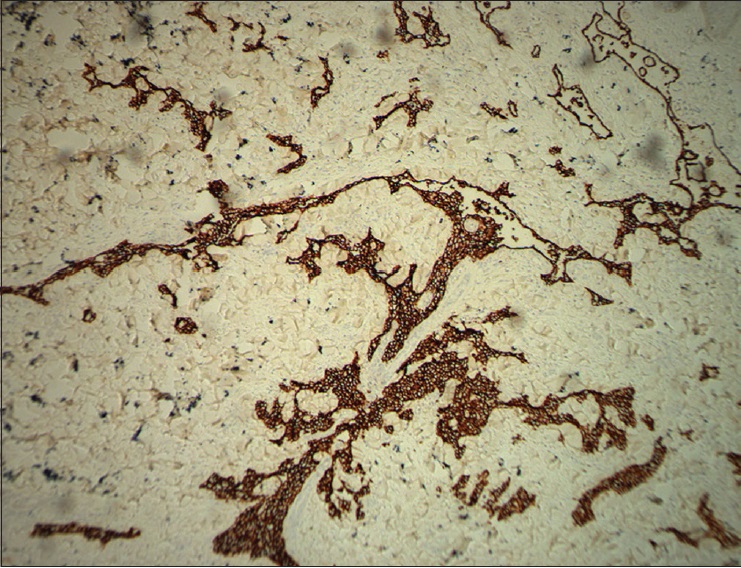 |
| Figure 3c: Immunohistochemical endothelial staining was positive for D2-40 (�200) |
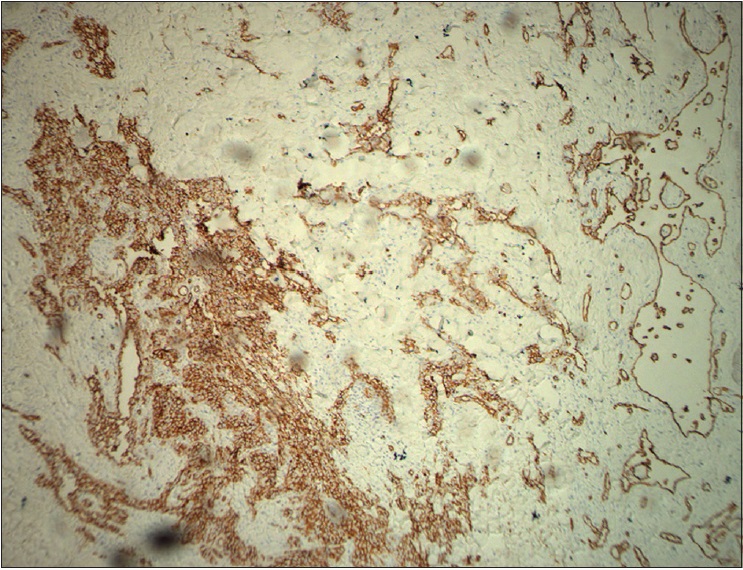 |
| Figure 3d: Immunohistochemical endothelial staining was positive for CD34 (×200) |
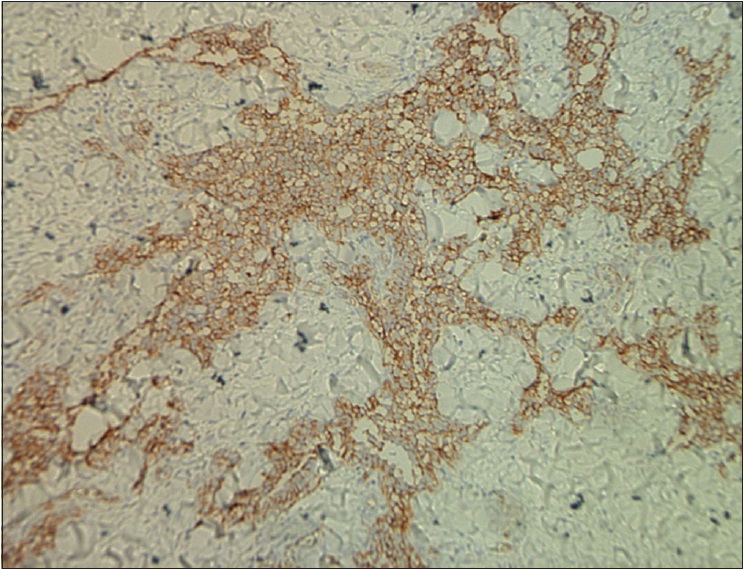 |
| Figure 3e: Immunohistochemical endothelial staining was positive for CD31 (×200) |
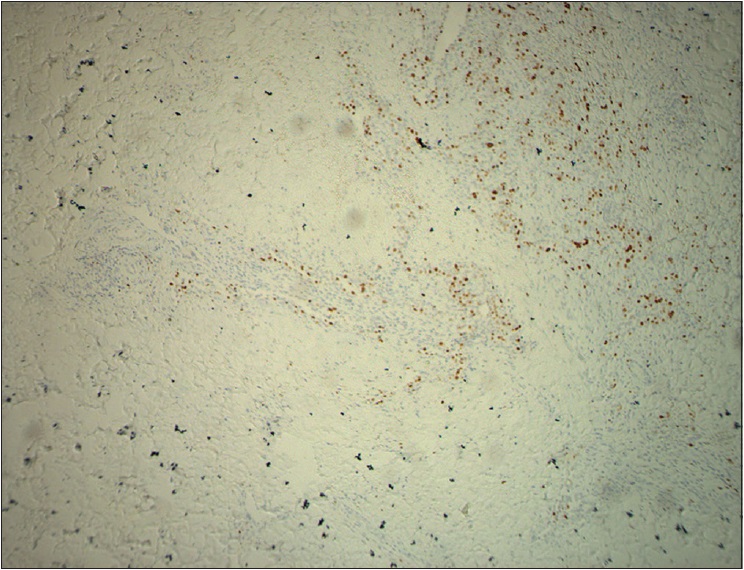 |
| Figure 3f: Immunohistochemical endothelial staining was positive for Ki-67 (�200) |
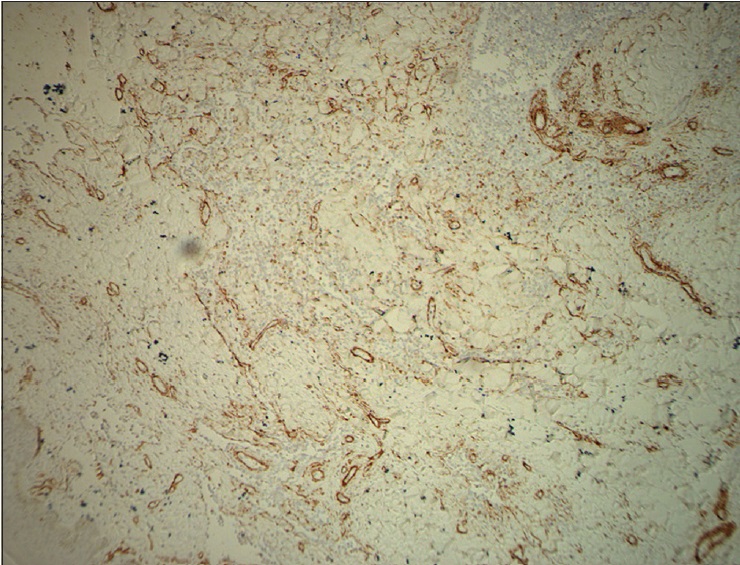 |
| Figure 3g: Smooth muscle actin was stained in the vessel wall but was negative in endothelial cells (×200) |
Retiform hemangioendothelioma is a rare, low-grade angiosarcoma, that was first reported by Calonje et al. in 1994.[1] It is most often found in the extremities, especially in the legs. The clinical features include slowly growing purple-red plaques or nodules, that are no larger than 3 cm in diameter. Retiform hemangioendothelioma usually occurs as a single lesion and slowly enlarges without symptoms, although some multifocal lesions have been reported. Local recurrence is common and distant metastases or tumor-related death rarely occurs.
The pathological characteristics of retiform hemangioendothelioma include poorly defined tumor tissue boundaries that extend into the dermis and/or subcutaneous tissue and long, arborizing vessels.[2] The vascular spaces are formed from monomorphic endothelial cells with apical nuclei and sparse cytoplasm which protrude into the lumen and create a hobnail appearance. A large number of lymphocytes that are closely related to endothelial cells are observed within the lumen and surrounding the vessels. Focally, intravascular papillae with collagenous cores are observed. A solid component consisting of bland spindle-shaped cells and some endothelial cells is observed in most tumors. Immunohistochemical endothelial staining is usually positive for the vascular markers CD31 and CD34. Because D2-40 is a recently described lymphatic marker, many previously reported cases of retiform hemangioendothelioma were not tested for this marker. Recently, D2-40 positivity has been demonstrated in rare cases of retiform hemangioendothelioma.[3],[4] However, the lymphatic origin of these tumors cannot be validated until more tumors with D2-40 are reported or more sophisticated markers of lymphatic differentiation become available to test these neoplasms.[4] The present case showed that in addition to expressing CD31 and CD34, retiform hemangioendothelioma also expresses D2-40. This case confirmed the possible lymphatic differentiation of retiform hemangioendothelioma.
The clinical characteristics and histological changes in retiform hemangioendothelioma and papillary intralymphatic angioendothelioma are similar and require further identification. Clinically, retiform hemangioendothelioma occurs mostly in middle-aged adults, whereas papillary intralymphatic angioendothelioma presents mainly in infants and children and it has been confirmed that the former is an adult variant of the latter. The clinical manifestations of both retiform hemangioendothelioma and papillary intralymphatic angioendothelioma include slow-growing plaques or nodules without symptoms, in the distal extremities. Histopathologically, papillary intralymphatic angioendothelioma does not exhibit a retiform pattern and is characterized by cavernous hemangioma-like vascular spaces with more prominent intravascular papillae with a collagenous core. Conventional angiosarcoma usually presents in a different clinical setting and is histologically characterized by focal pleomorphism and mitoses. Angiosarcoma exhibits multilayering of endothelial cells and hobnail endothelial cells are absent.
Local excision is the main treatment method for retiform hemangioendothelioma. However, because retiform hemangioendothelioma recurs frequently, it is advised to extend the surgical margins (3 cm). Radiotherapy and chemotherapy should be considered if the neoplasm is too large to excise, but the therapeutic effects require further investigation. Since the retiform hemangioendothelioma rarely metastasizes, a lymph node ultrasound examination is generally more beneficial than performing a sentinel lymph node biopsy.[5]
Here, we report a case of massive retiform hemangioendothelioma that expressed D2-40, a lymphatic differentiation marker. Clinically, most cases of retiform hemangioendothelioma are surgically resected. However, the case in our report exhibited diffuse damage and could not be surgically removed. No curative therapy exists. The patient eventually refused treatment due to her financial situation. As the patient refused further treatment, we could not explore the possible therapies to relieve her symptoms.
Acknowledgment
The authors thank Jing Li for her technical support and director Hualiang Xiao of pathology department for his assistance on the immunohistochemistry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Calonje E, Fletcher CD, Wilson-Jones E, Rosai J. Retiform hemangioendothelioma. A distinctive form of low-grade angiosarcoma delineated in a series of 15 cases. Am J Surg Pathol 1994;18:115-25. [Google Scholar] |
| 2. | Zhang G, Lu Q, Yin H, Wen H, Su Y, Li D, et al. A case of retiform-hemangioendothelioma with unusual presentation and aggressive clinical features. Int J Clin Exp Pathol 2010;3:528-33. [Google Scholar] |
| 3. | Emberger M, Laimer M, Steiner H, Zelger B. Retiform hemangioendothelioma: Presentation of a case expressing D2-40. J Cutan Pathol 2009;36:987-90. [Google Scholar] |
| 4. | Parsons A, Sheehan DJ, Sangueza OP. Retiform hemangioendotheliomas usually do not express D2-40 and VEGFR-3. Am J Dermatopathol 2008;30:31-3. [Google Scholar] |
| 5. | Hirsh AZ, Yan W, Wei L, Wernicke AG, Parashar B. Unresectable retiform hemangioendothelioma treated with external beam radiation therapy and chemotherapy: A case report and review of the literature. Sarcoma 2010;2010. pii: 756246. [Google Scholar] |
Fulltext Views
2,728
PDF downloads
1,115





