Translate this page into:
Matched case-control study to examine association of psoriasis and migratory glossitis in India
Correspondence Address:
Sanjay Singh
C-23, Swastik Towers, Lanka, Varanasi-221 005
India
| How to cite this article: Singh S, Nivash S, Mann BK. Matched case-control study to examine association of psoriasis and migratory glossitis in India. Indian J Dermatol Venereol Leprol 2013;79:59-64 |
Abstract
Background: Psoriasis is a multifactorial disease. Genetic and environmental factors, which determine the disease epidemiology and clinical spectrum, are heterogeneous in different populations. A few case-control studies from other countries have shown an association between psoriasis and migratory glossitis (MG). The characteristics of the association (e.g. relationship with gender, severity of psoriasis, early- versus late-onset psoriasis, etc.) have not been clearly defined. Aim: To investigate the association of psoriasis and MG by conducting a matched case-control study in India. Methods: The study was conducted on 600 patients with psoriasis and 800 age- and sex-matched controls. Patients were examined for oral lesions and psoriasis severity was assessed by overall severity index (OSI) and psoriasis area and severity index (PASI). We compared the proportions of patients and controls with oral lesions, proportions of male and female patients who had MG, psoriasis severity scores of patients with or without MG, and proportions of early- and late-onset psoriasis patients who had MG. Results: Significantly, more patients had oral lesions than controls (P=0.0013). There was a strong association between psoriasis and MG (P<0.0001). MG and fissured tongue (FT) occurring in the same patient were also strongly associated with psoriasis (P=0.0003). There was a weak association of psoriasis with FT (P=0.0456). Significantly, higher proportion of male patients had MG compared to female patients (P=0.0246). Patients with MG had more severe psoriasis compared to those without the tongue lesions (P<0.0001). Similar proportions of patients with type 1 and type 2 psoriasis had MG (P=0.7268). Conclusions: The results suggest that MG is a rare manifestation of psoriasis which occurs more commonly in male patients and in those with severe disease, and that it occurs with equal frequency in early- and late-onset psoriasis. It will be interesting to follow those patients who have MG, but not psoriasis, to see whether they develop psoriasis phenotype in future.Introduction
Psoriasis is a multifactorial disease; complex genetics and environmental factors interact to produce immunological aberrations which result in the psoriasis phenotype. Genetic susceptibility and environmental factors are not same everywhere, but are heterogeneous, being different in different geographical regions. Examples include heterogeneity in the genetic susceptibility for psoriasis in different populations [1],[2],[3],[4],[5] and even from geographically distant places in a large country like India. [6],[7] Even within the apparently more homogeneous subgroup of psoriasis vulgaris (chronic plaque psoriasis), heterogeneity related to human leukocyte antigen (HLA) has been demonstrated between disease of early-onset (before 40 years of age) and late-onset disease, [8] and further subdivisions [9] seem probable. [10]
Environmental factors are also obviously likely to differ among different populations. These differing genetic and environmental factors will determine the epidemiology and the clinical spectrum of a multifactorial disease like psoriasis.
Although there are numerous interventional studies, there is a general lack of observational studies to understand the epidemiology and clinical spectrum of psoriasis in different populations. Whenever such studies have been conducted, focus has been on finding the prevalence, which has been found to vary greatly. [11]
There are a few studies on association of psoriasis and benign migratory glossitis (MG) (synonym: migratory glossitis), some suggesting a lack of association, [12] whereas others showing an association. [13],[14],[15],[16],[17] Against this backdrop, we conducted a large case-control study to examine the association of MG and psoriasis in India and to determine the characteristics of the association with regard to gender, severity of psoriasis, and its early- versus late-onset.
Methods
This study was conducted on 600 patients with psoriasis and 800 controls between August 2009 and May 2012. Patients were selected consecutively from those attending the dermatology and venereology outpatient clinic of a tertiary hospital located in central part of India. The study was approved by the local Ethics Committee. Patients with all types of psoriasis were included; the only exclusion criterion was the use of any systemic antipsoriatic treatment in the past 1 month. Age- and sex-matched controls were selected from the healthy attendants accompanying other patients who did not have psoriasis. Thus, both the patients and the controls represented the same population.
All the patients included had typical clinical features of psoriasis, but in two cases that had lesions in linear distribution, skin biopsy and histopathological examination were done to confirm the diagnosis.
Psoriasis area and severity index (PASI) was calculated when appropriate. PASI was not calculated in patients who had lesions only in nails, scalp, palms and soles, or those who had only a few lesions of psoriasis. In addition, overall severity of psoriasis was assessed on a 0-10 scale (overall severity index [OSI]). Patients who had onset of disease before 40 years of age were considered to have type 1 (early-onset) psoriasis and those with later-onset type 2 (late-onset) psoriasis.
Oral examination was performed on both cases and controls and any abnormalities detected were recorded. MG was diagnosed on the basis of areas of erythema and loss of filiform papillae in geographical pattern and history of its migratory nature. Some of the patients had not noticed the lesions on tongue; in such cases, clinical photograph was taken and they were re-examined after 1-3 months. If the re-examination showed change in pattern of the lesions on tongue, only then these patients were recorded as having MG. Fissured tongue (FT) was diagnosed as anteroposteriorly oriented fissure, with or without branched fissures extending laterally.
We compared the two groups for age and gender, the proportion of patients and controls with oral lesions, proportion of male and female patients who had MG, psoriasis severity scores of patients with or without MG, and proportions of early- and late-onset psoriasis patients who had MG. The data was analyzed using an online statistical calculator (http://www.graphpad.com.) Unpaired t-test and Chi-square test were used as appropriate. The two-tailed P value of <0.05 was considered significant. Confidence intervals (CI) and odds ratios (OR) were calculated using online calculators (http://vassarstats.net and http://www.medcalc.org, respectively).
Results
The patients (n=600) and controls (n=800) were matched for age (patients vs. controls, Mean±SD, 34.07±17.3 years vs. 34.73±12.9 years, P=0.4132) and gender ratio (proportion of female, patients vs controls, 38.83% vs. 39%, P=0.9495).
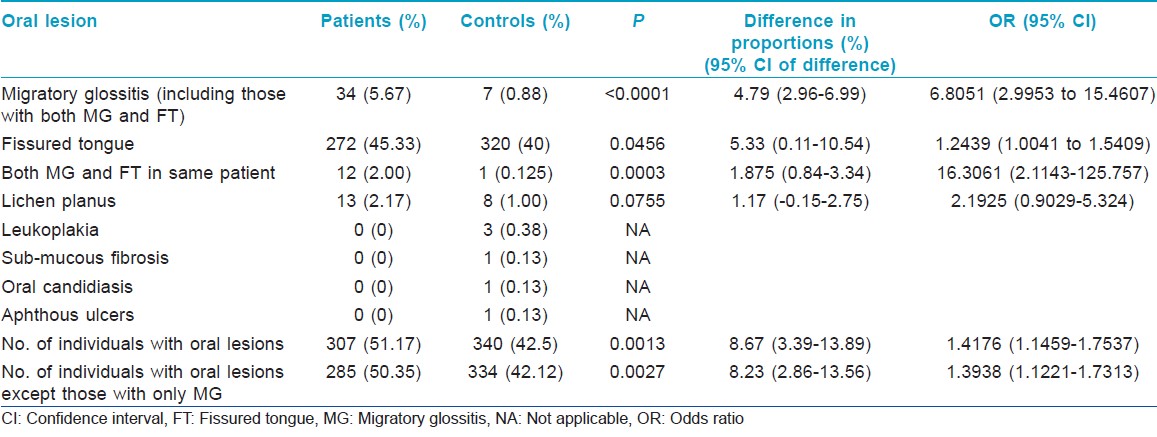
Significantly, more number of patients had oral lesions compared to controls [Table - 1]. There was a strongly significant association between psoriasis and MG [Figure - 1] and [Figure - 2]. Association between psoriasis and FT was weakly significant. When the patients and controls who had MG were excluded from analysis, the proportion of patients having oral lesions was still significantly higher compared to controls. Age (Mean±SD) of patients with MG (n=34) was 31.77±18.97 years and of controls with MG (n=7) was 42.43±15.43 years.
 |
| Figure 1: A child with palmar psoriasis, migratory glossitis, and fissured tongue |
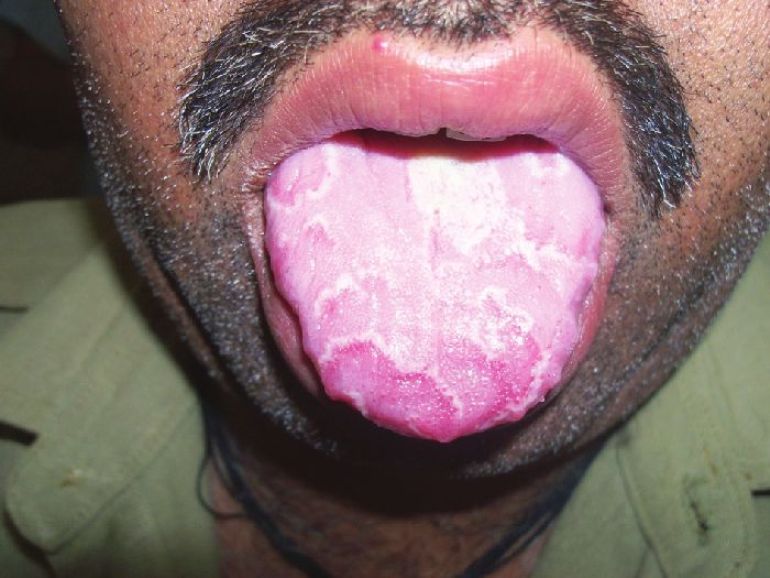 |
| Figure 2: Migratory glossitis in a middle-aged patient with psoriasis |
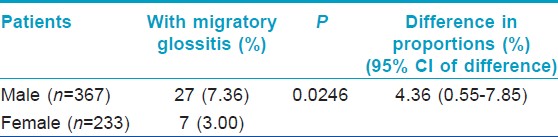
Significantly, higher proportion of male patients had MG compared to female patients [Table - 2].
Among the seven controls who had MG, there were five males (out of 488 male controls, 1.025%) and 2 females (out of 312 female controls, 0.641%), the difference in proportion (0.38%) was not significant (P=0.5699, 95% CI of difference, −1.38-1.81).
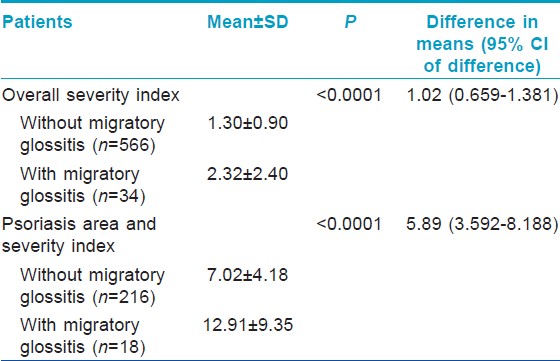
Severity of psoriasis as assessed by both OSI and PASI was significantly more in patients who had MG compared to those who did not [Table - 3].
Proportion of patients with type 1 psoriasis who had MG (24 of 439, 5.47%) was not significantly different from the patients with type 2 psoriasis having MG (10 of 161, 6.21%) (P=0.7268; difference in proportions, 0.74%; 95% CI of difference, −3.04-5.9).
Patients with different types of psoriasis who had MG were as follows: Palmoplantar psoriasis (12 of 254, 4.72%), chronic plaque psoriasis (15 of 251, 5.98%), scalp psoriasis (3 of 62, 4.84%), guttate psoriasis (0 of 9), nail psoriasis (0 of 8), psoriatic arthritis (0 of 7), psoriatic erythroderma (3 of 4), pustular psoriasis (1 of 2), flexural psoriasis (0 of 2), and genital psoriasis (0 of 1).
Discussion
Oral mucosa appears to be rarely involved in psoriasis, occasionally lip lesions or white oral lesions, especially in the buccal mucosa, or lesions clinically indistinguishable from geographical tongue (sometimes termed "annulus migrans" or "erythema circinatum"), particularly in generalized pustular psoriasis, have been reported. [18] MG is a benign inflammatory condition of the tongue of unknown etiology, which presents as map-like areas of erythema which are not constant in size, shape, or location. [18]
The aim of this study was to investigate the association of psoriasis with MG in India and to study the characteristics of this association in relation to the features of psoriasis like gender of the patient, severity, and early- versus late-onset of psoriasis. A further goal was to verify whether any other type of oral lesions are more frequently seen in patients with psoriasis versus controls. This study was conducted on 600 consecutively selected patients and age- and sex-matched controls (n=800). Patients with all types of psoriasis were included; the only exclusion criterion was the use of any systemic antipsoriatic treatment in past 1 month, which could have affected the appearance of MG. MG was diagnosed on the basis of presence of areas of erythema and loss of filiform papillae in geographical pattern and the history of its migratory nature. FT was diagnosed as anteroposteriorly oriented fissure, with or without branched fissures extending laterally. Other findings in oral mucosa were also recorded.
Significantly more number of patients had oral lesions compared to controls (P=0.0013); among the oral lesions, MG (P<0.0001) and MG and FT occurring in the same patient (P=0.0003) were significantly more common in patients as compared to the controls. Association of psoriasis with FT was weakly significant (P=0.0456). Proportion of patients with oral lesions was significantly more even after exclusion of cases and controls who had MG (P=0.0027). Significantly higher proportion of male patients had MG compared to the proportion of female patients (P=0.0246). Patients with MG had more severe psoriasis, as determined by the OSI and PASI, compared to those patients who did not have MG (P<0.0001). Proportions of patients with type I and type II psoriasis who had MG were not significantly different in this study (P=0.7268).
There are a limited number of studies focusing on oral lesions in psoriasis. Cross-sectional studies have given conflicting results with a few studies suggesting that geographic tongue is not an oral manifestation of psoriasis, [12] while others [19],[20],[21] suggesting that MG may be a manifestation of psoriasis. A few controlled studies have addressed this problem [Table - 4], which have tended to show that MG and FT are more frequently seen in patients with psoriasis compared to controls. Histopathological findings support the hypothesis that MG may be considered an expression of oral psoriasis. [22] Both in psoriasis and in benign MG, highly significant association with human leukocyte antigen (HLA)-Cw6 and weakly significant association with B13 have been shown. [23]
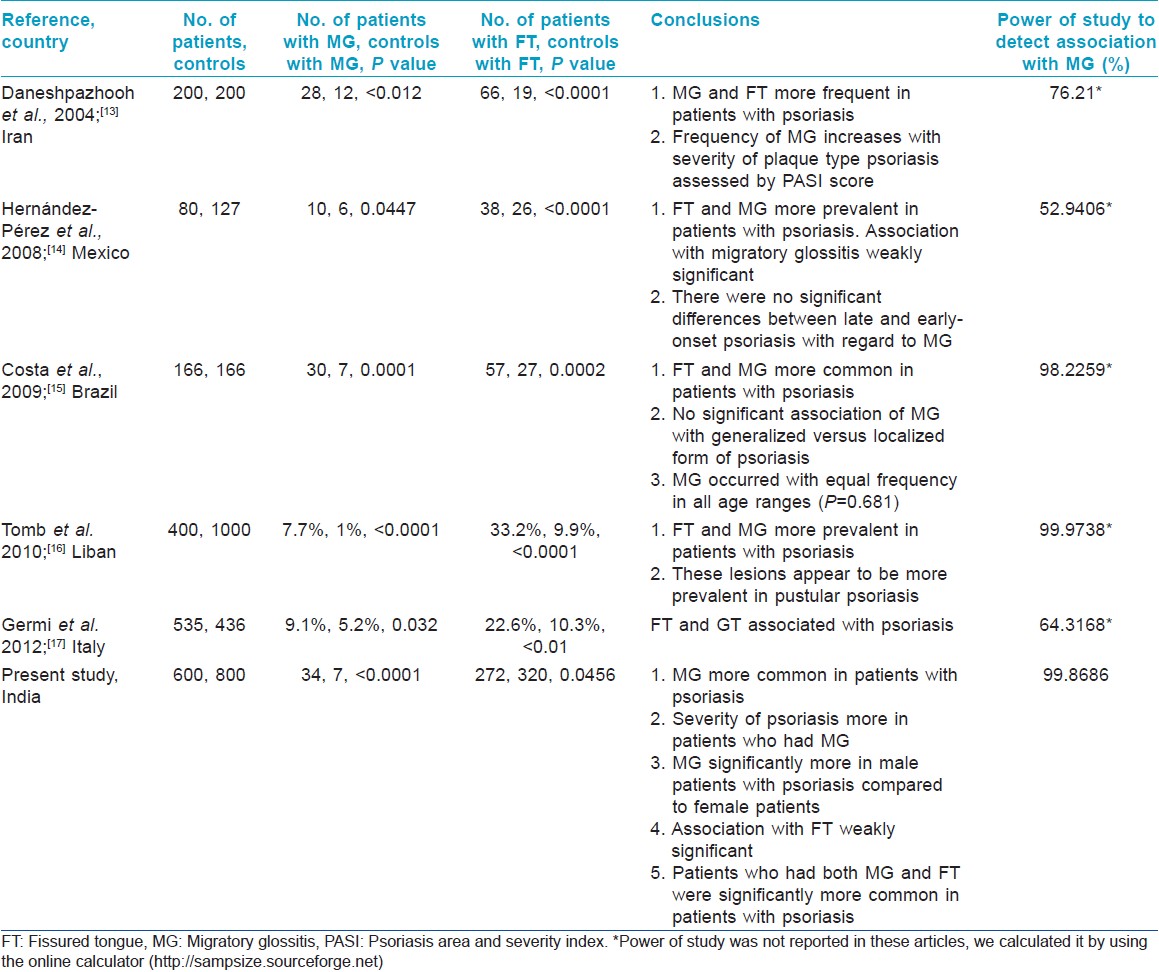
There is a general lack of observational studies on psoriasis to understand its epidemiology, clinical spectrum, and variations in its presentation in different geographical regions. As phenotype of psoriasis may vary depending on various heterogeneous genetic and environmental influences in different parts of the world, further studies in this area are likely to give important information.
This first case-control study from India on association of psoriasis and MG had a large sample size and high power. The study showed that the patients with psoriasis have a highly significant association with oral lesions and with MG, MG is more common in male patients compared to female patients, patients with MG have more severe psoriasis, similar proportions of patients with type 1 and type 2 psoriasis have MG, and that there is a weak association between FT and psoriasis. The results suggest that MG is a rare manifestation of psoriasis which occurs more commonly in male patients and in those with severe disease. It will be interesting to follow those patients who have MG, but not psoriasis, to see whether they develop psoriasis phenotype in future.
| 1. |
Chandran V. The Genetics of Psoriasis and Psoriatic Arthritis. Clin Rev Allergy Immunol 2012; Jan 25. In Press
[Google Scholar]
|
| 2. |
Gandhi G, Buttar BS, Albert L, Hasan Q, Aggarwal RK. Psoriasis-associated genetic polymorphism in North Indian population in the CCHCR1 gene and in a genomic segment flanking the HLA-C region. Dis Markers 2011;31:361-70.
[Google Scholar]
|
| 3. |
Chiu HY, Huang PY, Jee SH, Hu CY, Chou CT, Chang YT, et al. HLA polymorphism among Chinese patients with chronic plaque psoriasis: Subgroup analysis. Br J Dermatol 2012;166:288-97.
[Google Scholar]
|
| 4. |
Al-Mamari F, Al-Shirawi A, Banodkar D, Al-Hashmi S, Al-Yahyaae F, Varghese M, et al. HLA antigens in Omani Psoriasis Vulgaris Patients. Oman Med J 2009;24:27-9.
[Google Scholar]
|
| 5. |
Valdimarsson H. The genetic basis of psoriasis. Clin Dermatol 2007;25:563-7.
[Google Scholar]
|
| 6. |
Singh S, Singh U, Singh S. Human leukocyte antigen in patients with psoriasis. Indian J Dermatol Venereol Leprol 2011;77:535.
[Google Scholar]
|
| 7. |
Umapathy S, Pawar A, Mitra R, Khuperkar D, Devaraj JP, Ghosh K, et al. HLA-A and HLA-B alleles associated in psoriasis patients from Mumbai, Western India. Indian J Dermatol 2011;56:497-500.
[Google Scholar]
|
| 8. |
Henseler T, Christophers E. Psoriasis of early and late onset: Characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985;13:450-6.
[Google Scholar]
|
| 9. |
Griffiths CE, Christophers E, Barker JN, Chalmers RJ, Chimenti S, Krueger GG, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007;156:258-62.
[Google Scholar]
|
| 10. |
Capon F, Burden AD, Trembath RC, Barker JN. Psoriasis and other complex trait dermatoses: From Loci to functional pathways. J Invest Dermatol 2012;132:915-22.
[Google Scholar]
|
| 11. |
Griffiths CE, Barker JN. Psoriasis. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8 th ed. Chichester: Wiley-Blackwell; 2010. p. 20.1-20.60.
th ed. Chichester: Wiley-Blackwell; 2010. p. 20.1-20.60.'>[Google Scholar]
|
| 12. |
Miloðlu O, Göregen M, Akgül HM, Acemoðlu H. The prevalence and risk factors associated with benign migratory glossitis lesions in 7619 Turkish dental outpatients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:e29-33.
[Google Scholar]
|
| 13. |
Daneshpazhooh M, Moslehi H, Akhyani M, Etesami M. Tongue lesions in psoriasis: A controlled study. BMC Dermatol 2004;4:16.
[Google Scholar]
|
| 14. |
Hernández-Pérez F, Jaimes-Aveldañez A, Urquizo-Ruvalcaba Mde L, Díaz-Barcelot M, Irigoyen-Camacho ME, Vega-Memije ME, et al. Prevalence of oral lesions in patients with psoriasis. Med Oral Patol Oral Cir Bucal 2008;13:E703-8.
[Google Scholar]
|
| 15. |
Costa SC, Hirota SK, Takahashi MD, Andrade H Jr, Migliari DA. Oral lesions in 166 patients with cutaneous psoriasis: A controlled study. Med Oral Patol Oral Cir Bucal 2009;14:e371-5.
[Google Scholar]
|
| 16. |
Tomb R, Hajj H, Nehme E. Oral lesions in psoriasis. Ann Dermatol Venereol 2010;137:695-702.
[Google Scholar]
|
| 17. |
Germi L, De Giorgi V, Bergamo F, Niccoli MC, Kokelj F, Simonacci M, et al. Psoriasis and oral lesions: Multicentric study of Oral Mucosa Diseases Italian Group (GIPMO). Dermatol Online J 2012;18:11.
[Google Scholar]
|
| 18. |
Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8 th ed. Chichester: Wiley-Blackwell; 2010. p. 69.1-69.129.
th ed. Chichester: Wiley-Blackwell; 2010. p. 69.1-69.129.'>[Google Scholar]
|
| 19. |
Buchner A, Begleiter A. Oral lesions in psoriatic patients. Oral Surg Oral Med Oral Pathol 1976;41:327-32.
[Google Scholar]
|
| 20. |
Pogrel MA, Cram D. Intraoral findings in patients with psoriasis with a special reference to ectopic geographic tongue (erythema circinata). Oral Surg Oral Med Oral Pathol 1988;66:184-9.
[Google Scholar]
|
| 21. |
Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatol 2006;31:192-5.
[Google Scholar]
|
| 22. |
Femiano F. Geographic tongue (migrant glossitis) and psoriasis. Minerva Stomatol 2001;50:213-7.
[Google Scholar]
|
| 23. |
Gonzaga HF, Torres EA, Alchorne MM, Gerbase-Delima M. Both psoriasis and benign migratory glossitis are associated with HLA-Cw6. Br J Dermatol 1996;135:368-70.
[Google Scholar]
|
Fulltext Views
4,526
PDF downloads
2,117





