Translate this page into:
Measurement of vitamin D and cathelicidin (LL-37) levels in patients of psoriasis with co-morbidities
2 Department of Dermatology, Farwaniya Hospital, Kuwait
Correspondence Address:
Nawaf Al-Mutairi
280, Farwaniya 80000
Kuwait
| How to cite this article: Al-Mutairi N, EL Eassa B, Nair V. Measurement of vitamin D and cathelicidin (LL-37) levels in patients of psoriasis with co-morbidities. Indian J Dermatol Venereol Leprol 2013;79:492-496 |
Abstract
Background: During the last decade, a lot of co-morbidities (diabetes, obesity, heart disease, etc.) have been described to be associated with psoriasis, but the exact link at the molecular level is not well-known. Researchers have shown molecular level changes in vitamin D pathway and its relationship to cathelicidin. Aims: To estimate the levels of cathelicidin (LL-37), and vitamin D in psoriasis patients with co-morbidities, and compare them with matched healthy controls. Methods: One hundred consecutive patients with stable plaque psoriasis (psoriasis area and severity index ≥10) with no systemic treatment in the past 3 months were investigated for the serum levels of vitamin D and LL-37, and compared with equal number of matched healthy volunteers. Results: The serum vitamin D levels were significantly lower in patients. Furthermore, the levels of serum LL-37were significantly high. Conclusion: Our study showed that the low serum levels of vitamin D, and higher blood levels of cathelicidin could form a molecular level clue in the pathogenesis of psoriasis patients, who are more likely to develop co-morbidities.Introduction
Psoriasis is a common chronic inflammatory skin disease, with over 2-3% of the world population suffering from psoriasis. [1] Although, the exact pathogenesis of this disease is still not completely understood, it is characterized by dysregulation of cutaneous innate immunity. [2] Various cytokines, chemokines, antimicrobial peptides (AMP) are found in high-levels in psoriatic plaques. [3] Psoriasis is not just one disease, it is turning out to be a syndrome having significant associations with many chronic diseases. Increasingly large numbers of studies have been published, regarding the co-morbidities associated with psoriasis. [4],[5],[6],[7] Furthermore, lot of research is going on at molecular level to fight chronic conditions such as heart disease, diabetes, obesity, and metabolic syndrome. And, some of the molecular level changes seen in these conditions have also been observed in patients of psoriasis. [8] Hence, it would be interesting to find a link between psoriasis and co-morbidities at molecular level. Recently vitamin D pathway has been extensively studied, and vitamin D receptors (VDR) have been found to play a significant role in diabetes mellitus, heart disease, malignancies etc. [9] and hence, we decided to study the interplay of vitamin D levels, and AMP cathelicidin (LL-37) in patients with psoriasis associated with co-morbidities, and compared them with age and sex matched healthy controls.
Methods
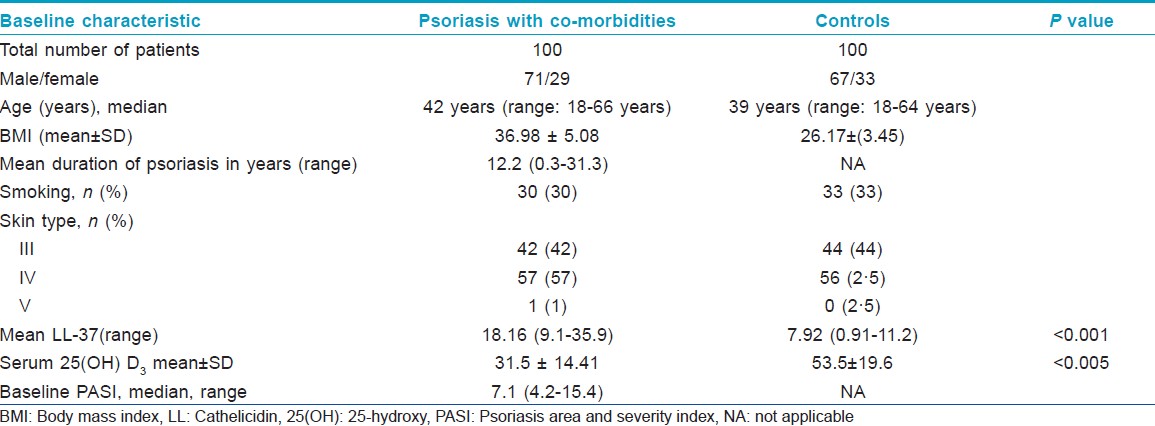
One hundred consecutive eligible patients (71 male and 29 female) of age above 18 years were enrolled in this study. They had at least 10% body surface area affected by stable plaque psoriasis (Group A), having an associated co-morbid condition like diabetes, obesity, heart disease, hypertension, and/or metabolic syndrome. These patients were seen in Dermatology Clinic of Farwaniya Hospital between January 2010 and March 2012 [Table - 1]. This work was approved by the Ethics Committee of Farwaniya Hospital. Equal number of healthy gender and age matched subjects were also recruited as controls (Group B). All these subjects were included in the study after obtaining informed voluntary consent. They were of Fitzpatrick skin types III to V and, none of them had received any form of systemic therapy for psoriasis or vitamin D supplementation for at least 3 months prior to the study. The psoriasis disease severity was evaluated and was graded according to the area affected by psoriasis area and severity index (PASI) scoring system. The PASI score of all patients was determined by one dermatologist. Data was collected from all the cases, which included, age, sex, gender, weight, height, body mass index (BMI), waist circumference, blood pressure (BP), smoking habit, age at onset of psoriasis, severity of psoriasis, presence and distribution of psoriatic arthropathy and concomitant systemic diseases (co-morbidities). To determine waist circumference, we located the upper hip bone and placed a measuring tape at the level of the uppermost part of the hipbone around the abdomen (ensuring that the tape measure was horizontal).
All investigations including, blood sugar, lipid profile, liver function test, and kidney function test were carried out for all the patients and controls. The reference values used for defining the co-morbidities were as follows: Hypertension (BP ≥140/90 mm of Hg);diabetes mellitus (fasting blood sugar >125 mg%); dyslipidemia (triglyceride ≥150 mg%, and/or low-density lipoprotein cholesterol ≥160 mg%, and/or high-density lipoprotein (HDL) cholesterol<40 mg% in males and <50 mg% in females); obesity (BMI>30 kg/m 2 ). Metabolic syndrome was diagnosed in the presence of three or more criteria of the National Cholesterol Education Program′s Adult treatment Panel III (ATP III): hypertriglyceridemia ≥150 mg/dL (>1.7 mmol/l); HDL cholesterol<40 mg/dL(<1.0 mmol/l) in men or <50 mg/dL (<1.3 mmol/l) in women; BP>135/85 mm of Hg; fasting plasma glucose >100 mg/dL); waist circumference>102 cm in men or >88 cm in women. [10]
Blood samples were taken from all the subjects for estimation of levels of vitamin Dand human cathelicidin peptide, LL-37. Vitamin D was measured using competitive enzyme-immunoassay technique with a selected monoclonal antibody recognizing 25-hydroxy (OH) vitamin D. Standards; non-specific binding, controls, and patient samples which were assayed for 25 (OH) vitamin D were incubated after the extraction step with the detection antibody. The pre-incubated solution was then transferred to a microplate coated with 25 (OH) vitamin D. During this incubation step, 25 (OH) vitamin D in the sample and a fixed amount of 25 (OH) vitamin D bound to the microtiter well competed for the binding of the detection antibodies. Then a peroxidase-conjugated anti-mouse antibody was added into each microplate well and a complex of 25(OH) vitamin D-detection antibody-peroxidase conjugate was formed. Tetramethylbenzidine was used as a peroxidase substrate. Finally, an acidic stop solution was added to terminate the reaction.
The cathelicidin, LL-37 was measured in serum by solid phase enzyme linked immunoassay (ELISA) using a commercial assay with aminimum concentration, which could be measured (limit of detection [LOD] of 0.14 ng/mL), and measurable concentration range of 0.14-100 ng/ml. (Hycult Biotechnology, Uden, The Netherlands).
The generated results were analyzed by SPSS (SPSS 17.0, SPSS Inc., Chicago, IL). Data reported as medians (range, minimum to maximum). Comparison of laboratory parameters between and within the two groups (t-tests and ANOVA) was carried out to identify the biochemical inter relationship between the patients and normal controls.
Results
The median age was 42 years (range:18-66 years) in the psoriasis with co-morbidities group, and 39 years (range: 18-64 years) in the healthy control group.The median duration of psoriasis was 12.2 years (range: 0.3-31.3 years). Both the groups were similar in distribution for gender and age. Co-morbid conditions that existed in our patients were hypertension, diabetes, cardiovascular disease, and metabolic syndrome as given in [Table - 2].

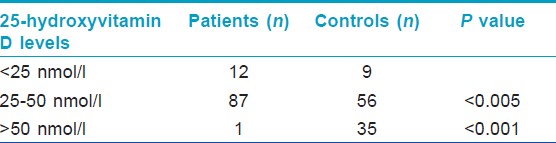
The mean ± SD serum 25(OH) vitamin D concentration was 29·53 nmol/L ± 9·38 nmol/L in patients, and 53.5 nmol/L± 19.6 nmol/L in healthy controls. The difference was statistically significant (P < 0.0001). Eighty seven (87%) patients with psoriasis, and 56 (56%) healthy controls had low vitamin D levels (serum levels of 25 [OH] vitamin D < 50 nmol/L). Of these subjects, 12 with psoriasis and nine healthy controls had vitamin D deficiency (serum levels of 25 (OH) vitamin D <25 nmol/L) [Table - 3].
Serum LL-37 levels were above the LOD of ELISA kit in all psoriasis samples (sensitivity 0.14 ng/ml). Analysis of cathelicidin levels revealed significantly elevated levels of cathelicidin in psoriasis patients compared with the healthy control group. The median serum LL-37 levels were significantly higher in psoriasis patients versus healthy controls (18.16 ng/mlvs. 7.92 ng/ml).We tested the association of BMI with LL-37 and it showed no statistically significant relationship (P = 0.18).
Discussion
A significant proportion of research carried out on psoriasis in the last decade has focused on co-morbidities and other conditions associated with it. Psoriasis has been linked to obesity, diabetes, heart disease, metabolic syndrome, hypertension, and various other conditions, and the list keeps on increasing. While the link has been more or less established beyond doubt, their origin is less known. [11] Various studies have shown that the individuals with severe psoriasis have an increased risk of heart attack, and this risk is independent of other major risk-factors like hypertension, diabetes, obesity, dyslipidemia, and smoking, which is also common in psoriasis. [12] Futhermore, recent studies [13] have suggested that people with severe psoriasis have a 50% higher mortality risk, and they die 3-6 years younger than those without psoriasis. These observational studies although, are helpful in generating a hypotheses, but are limited by the fact that they are unable to generate results that allow researchers to differentiate clearly between association and causality. The chances of observing a causal relation increase when there is a clear biological explanation for the association, the association is confirmed in multiple studies, and there is a dose-response relationship and a clear temporal relationship between exposure and outcome. The possible link between psoriasis and many of the associated diseases may be the presence of chronic inflammation and, in particular, elevated levels of the multifunctional cytokine tumor necrosis factor-α. It has been hypothesized that elevated levels of tumor necrosis factor-α are a biological explanation for few of the observed associations, which have shown chronic inflammation. [14] However, several other factors may play important roles and confound this association.
Recently, vitamin D has been shown to be playing an essential role in many conditions, apart from its well established link in the calcium metabolism and bone health. Recent largest to date study carried out in Denmark [15] showed that low-levels of vitamin D are associated with a markedly higher risk of heart attack and early death. In this study, the authors observed 40% higher risk of ischemic heart disease, 64% higher risk of heart attack, 57% higher risk of early death, and no less than 81% higher risk of death from heart disease even after adjustment for several factors that can influence vitamin D levels, and the risk of disease and death. Similarly, there are studies available in the literature, which have suggested a similar inverse relationship with obesity, [16] diabetes, [17] metabolic syndrome, [18] and hypertension. [19]
On the other hand, studies [8],[20] carried out on psoriasis patients have also shown deficiency of serum concentration of 25 (OH) vitamin D in these patients, and the evidence is growing. Furthermore, epidermal keratinocytes produce and secrete AMP that subsequently forms a biological guard on the skin surface. Dysfunction of these peptides has been involved in the pathogenesis of many inflammatory skin diseases. [21] Around 30 cathelicidin members have been identified in mammals, and one cathelicidin, named human cationic antibacterial protein of 18kDa, has been identified in humans. Its mature antibacterial peptide, LL-37, was shown to be expressed by keratinocytes in inflamed skin. [22] The cathelicidin, LL-37 is overexpressed in inflamed skin in psoriasis. [21],[22] Recently, the vitamin D pathway was identified as a regulator of cathelicidin expression in man. [23] Cathelicidin expression is directly regulated through vitamin D 3 , in which epigenetic changes such as histone acetylation can be activated or coactivators of the vitamin D 3 could be targeted. [24],[25],[26],[27] In co-activator pathway 1, 25 D 3 binds with VDR which subsequently heterodimerizes with the retinoid X receptor. This complex enhances the secretion of cathelicidin. [28],[29],[30],[31],[32] Schauberand Gallo [27] demonstrated that 1a, 25 (OH) 2 D 3 directly induced cationic AMP gene expression in a variety of tissue and cell type.The recent identification of the cationic AMP cathelicidin as a vitamin D target gene [25] and of cytochrome P450, family 27, subfamily B, polypeptide 1 (CYP27B1) gene and VDR upregulation in monocytes as the link between toll-like receptor-2 (TLR-2) activation on the one hand and cathelicidin production and intracellular mycobacteria killing on the other hand, a previously unknown and unexpected link between innate immunity and the vitamin D system has been created. [20] Lande et al. [33] showed that in patients of psoriasis, cathelicidin (LL-37) initiates an autoimmune response by activating TLR signaling in plasmacytoid dendritic cells (pDCs) of skin. It forms condensed aggregates by binding directly to DNA in pDCs, which are then presented to TLR-9 receptors. These activated pDCs secrete large amount of interferon α to lead to a T cell mediated immune response in psoriatic skin.
Our psoriasis group patients showed reduced level of circulating 25 (OH) vitamin D, with over-expression of cathelicidin LL-37 level, in contrast with the healthy control group. These findings show the existence of inverse relationship between vitamin D and LL-37 in psoriasis patients with associated co-morbidities. The limitation of our study was that it was not a blinded study, and some of the confounding factors like amount of sun exposure, or clothing habits could have an influence on the results.
Conclusion
From the results of our study and also keeping in mind the findings of various studies carried out in the past, it seems that vitamin D and not cathelicidin, may be the main factor in the pathogenesis of psoriasis. The levels of AMP cathelicidin, LL-37seems to be controlled by vitamin D. However, much more needs to be carried out to prove this hypothesis. It would be interesting to measure the cathelicidin levels in vitamin D deficient individuals without any chronic illness, associated co-morbid condition, or psoriasis.
| 1. |
Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol 2001;15:16-7.
[Google Scholar]
|
| 2. |
Mrowietz U, Reich K. Psoriasis-New insights into pathogenesis and treatment. Dtsch Arztebl Int 2009;106:11-9.
[Google Scholar]
|
| 3. |
Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 2012;39:225-30.
[Google Scholar]
|
| 4. |
Gladman DD. Biomarkers for comorbidities in psoriasis: A report from the grappa 2011 annual meeting. J Rheumatol 2012;39:2193-5.
[Google Scholar]
|
| 5. |
Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: Results from the National Psoriasis Foundation surveys 2003-2011. Dermatology 2012;225:121-6.
[Google Scholar]
|
| 6. |
Onumah N, Kircik LH. Psoriasis and its comorbidities. J Drugs Dermatol 2012;11:s5-10.
[Google Scholar]
|
| 7. |
Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: An experience from the Middle East. J Dermatol 2010;37:146-55.
[Google Scholar]
|
| 8. |
Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J Am Acad Dermatol 2012;67:931-8.
[Google Scholar]
|
| 9. |
Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005;26:662-87.
[Google Scholar]
|
| 10. |
National Institute of Health. Third report of the National Cholesterol Education Program Export Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary 2001. NIH pub no. 01-3670. Bethesda, MD: National Institute of Health, National Heart, Lung and Blood Institute. September 2002; pII-27.
[Google Scholar]
|
| 11. |
Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. J Invest Dermatol 2009;129:1601-3.
[Google Scholar]
|
| 12. |
Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: A systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:1931-42.
[Google Scholar]
|
| 13. |
Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: Results from a population-based study. Arch Dermatol 2007;143: 1493-9.
[Google Scholar]
|
| 14. |
Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatolog Treat 2008;19:5-21.
[Google Scholar]
|
| 15. |
Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol 2012;32:2794-802.
[Google Scholar]
|
| 16. |
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690-3.
[Google Scholar]
|
| 17. |
Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002;51:2294-300.
[Google Scholar]
|
| 18. |
Yin X, Sun Q, Zhang X, Lu Y, Sun C, Cui Y, et al. Serum 25(OH)D is inversely associated with metabolic syndrome risk profile among urban middle-aged Chinese population. Nutr J 2012;11:68.
[Google Scholar]
|
| 19. |
Dorjgochoo T, OuShu X, Xiang YB, Yang G, Cai Q, Li H, et al. Circulating 25-hydroxyvitamin D levels in relation to blood pressure parameters and hypertension in the Shanghai Women's and Men's Health Studies. Br J Nutr 2012;108:449-58.
[Google Scholar]
|
| 20. |
Liu JL, Zhang SQ, Zeng HM. ApaI, BsmI, FokI and TaqI polymorphisms in the vitamin D receptor (VDR) gene and the risk of psoriasis: A meta-analysis. J Eur Acad Dermatol Venereol 2012; Apr 28. doi: 10.1111/j.1468-3083.2012.04553.x. [Epub ahead of print].
[Google Scholar]
|
| 21. |
Dombrowski Y, Schauber J. Cathelicidin LL-37: A defense molecule with a potential role in psoriasis pathogenesis. Exp Dermatol 2012;21:327-30.
[Google Scholar]
|
| 22. |
Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997;272:15258-63.
[Google Scholar]
|
| 23. |
Dombrowski Y, Peric M, Koglin S, Ruzicka T, Schauber J. Control of cutaneous antimicrobial peptides by vitamin D3. Arch Dermatol Res 2010;302:401-8.
[Google Scholar]
|
| 24. |
Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004;173:2909-12.
[Google Scholar]
|
| 25. |
Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005;19:1067-77.
[Google Scholar]
|
| 26. |
Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Törmä H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol 2005;124:1080-2.
[Google Scholar]
|
| 27. |
Schauber J, Gallo RL. The vitamin D pathway: A new target for control of the skin's immune response? Exp Dermatol 2008;17:633-9.
[Google Scholar]
|
| 28. |
Schauber J, Oda Y, Büchau AS, Yun QC, Steinmeyer A, Zügel U, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 2008;128:816-24.
[Google Scholar]
|
| 29. |
Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol 2010;130:1297-306.
[Google Scholar]
|
| 30. |
Segaert S. Vitamin D regulation of cathelicidin in the skin: Toward a renaissance of vitamin D in dermatology? J Invest Dermatol 2008;128:773-5.
[Google Scholar]
|
| 31. |
Zelezetsky I, Pontillo A, Puzzi L, Antcheva N, Segat L, Pacor S, et al. Evolution of the primate cathelicidin. Correlation between structural variations and antimicrobial activity. J Biol Chem 2006;281:19861-71.
[Google Scholar]
|
| 32. |
Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: Dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol 2005;174:4271-8.
[Google Scholar]
|
| 33. |
Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 2011;3:82ra38.
[Google Scholar]
|
Fulltext Views
3,908
PDF downloads
2,145





