Translate this page into:
Mesotherapy with bicalutamide for female pattern hair loss
Corresponding author: Dr. Raquel de Melo Carvalho, Department of Dermatology, UNESP Botucatu, São Paulo, Brazil. raqueldemelocarvalho@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Carvalho RM, de Brito FX, Ramos PM, Machado CJ, Cortez de Almeida RF, Vaño-Galvan S et al. Mesotherapy with bicalutamide for female pattern hair loss. Indian J Dermatol Venereol Leprol. 2025;91:255-7. doi: 10.25259/IJDVL_99_2024
Dear Editor,
Female pattern hair loss (FPHL) is a prevalent condition that can have a significant impact on the quality of life. It has a fairly limited therapeutic arsenal and the outcomes often do not meet the patient’s expectations.1 Currently, the only drug approved by the FDA for FPHL is topical minoxidil.2
Recently, oral bicalutamide, a selective antagonist of androgen receptors (AR), has been used off-label to treat FPHL. However, although infrequent, some patients may present systemic adverse effects such as an increase in transaminase levels.3,4
Mesotherapy is a minimally invasive procedure that involves injecting therapeutic agents 2–4 mm into the skin.5 Mesotherapy using dutasteride or minoxidil has been proposed as an effective option to elicit hair regrowth and reduce systemic absorption.6 As far as we know, mesotherapy with bicalutamide has been little studied for FPHL.7
This pilot prospective study aimed to evaluate the efficacy and safety of mesotherapy with bicalutamide 0.5% in the treatment of FPHL as monotherapy.
We enrolled four Latin-American women with FPHL and Fitzpatrick skin phototypes ranging from II–IV. We included healthy pre-menopausal female patients diagnosed with FPHL, aged between 31 and 47 (mean 38.5) years in the study. Patients who underwent previous treatment for hair loss (last 3 months) and those with liver disease, other causes of hair loss, or on hair loss therapy were excluded. A written informed consent was obtained from the subjects to publish their photos and details of the cases. This study complies with the internationally accepted standards for research practice and reporting.
Each patient received five monthly intradermal injections of 2 mL bicalutamide 0.5% (skypharma®). Figure 1 demonstrates the treatment plan used in the study. The images were then appraised by three independent and experienced dermatologists who compared each of the T0 images with the T24 ones. They needed to achieve agreement using a five-point comparison scale: great worsening (−2), slight worsening (−1), no change (0), slight improvement (+1), and great improvement (+2).
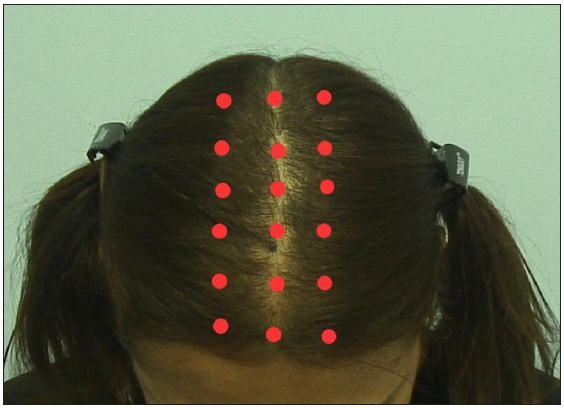
- Treatment plan: approximately 0.1 cc was injected into each red dot represented in the figure with a threaded syringe and 31 g needle at a 45-degree angle.
The clinical and trichoscopic images revealed stabilisation in the frontal area for two patients and improvement for one. Conversely, the mid scalp and vertex exhibited stabilisation in all cases (n = 4). The results are described in detail in Table 1 and Clinical and trichoscopic pictures were captured at baseline (T0) and 24 weeks (T24) using Fotofinder® technology are demonstrated in Figures 2 and 3.
| Variables | Statistics/categories | Obtained values |
|---|---|---|
| Sinclair Scale stage of female pattern hair loss: n (%) | 1 | 0 (0.0) |
| 2 | 3 (75.0) | |
| Presence of the conditions/disease (T0): n (%) | Acne | 2 (50.0) |
| Telogen Effluvium | 2 (50.0) | |
| Hirsutism | 1 (25.0) | |
| Obesity | 2 (50.0) | |
| Polycystic ovary syndrome | 0 (0.0) | |
| Values in pain scale (1–10): n (%) | 1–4 | 0 (0.0) |
| 5 | 1 (0.0) | |
| 6 and 7 | 0 (0.0) | |
| 8 | 3 (75.0) | |
| 9 and 10 | 0 (0.0) | |
| Side effects: n (%) | 0 (0.0) | |
| Self-report of seborrhea: n (%) | ||
| - Before treatment (T0) | -- Absent | 0 (0.0) |
| -- Mild | 2 (0.0) | |
| -- Intense | 2 (0.0) | |
| - Change in Self-report of seborrhea (T0–T6) | -- Worsening | 0 (0.0) p = 0.157(1) |
| -- Stabilisation | 2 (50.0) | |
| -- Slight improvement | 2 (50.0) | |
| -- Marked improvement | 0 (0.0) | |
| Categories of change in hair density (T0–T6)(2) | ||
| -- Worsening | 1 (25.0) p = 0.368(2) | |
| -- Stabilisation | 2 (50.0) | |
| - Frontal | -- Improvement | 1 (25.0) |
| - Mid scalp | -- Worsening | 0 (0.0) p > 0.999(2) |
| -- Stabilisation | 4 (100.0) | |
| -- Improvement | 0 (0.0) | |
| - Vertex | -- Worsening | 0 (0.0) p > 0.999(2) |
| -- Stabilisation | 4 (100.0) | |
| -- Improvement | 0 (0.0) | |
Notes: (1)score values for change in hair density were chosen as the most frequent (modal value) among the three types of evaluations conducted for each of the studied sides. The scale used varies from −2 to 2 (−2 = significant worsening, −1 = mild worsening, 0 = stabilisation, 1 = improvement, 2 = significant improvement); (2)p values obtained based on symmetry command (Stata/SE 12.1 for Windows) which performs exact symmetry (homogeneity) test in studies where non-independence holds (before-after).
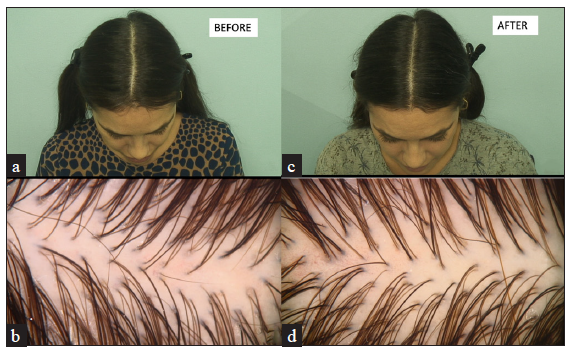
- Comparative analysis of the patient’s scalp condition (a, b) before treatment and (c, d) after treatment. Images (a) and (c) represent macroscopic views, while images (b) and (d) show trichoscopic findings. Post-treatment images demonstrate increased hair density indicating improvement compared to baseline.
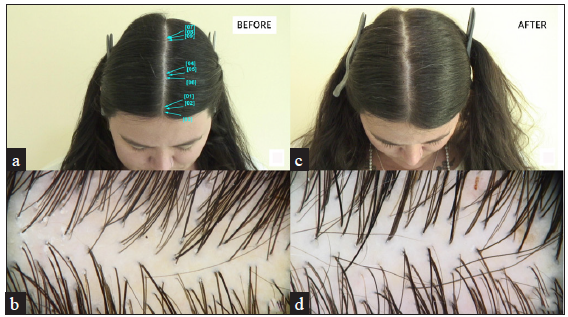
- Comparative analysis of the patient’s scalp condition (a, b) before treatment and (c, d). after treatment. Images (a) and (c) represent macroscopic views, while images (b) and (d) show trichoscopic findings. Post-treatment images demonstrate increased hair density indicating improvement compared to baseline.
There were no major side effects or complications following the procedure except for local pain, ecchymosis, and transitory edema. The pain was rated up to 8 (75.0%) on a scale from 0 to 10. With regard to the symptoms pre-intervention, half of the patients had mild and half had intense seborrhoea at T0. Among those with intense seborrhoea, there was mild improvement reported at T24.
Mesotherapy has emerged as a popular minimally invasive procedure for non-scarring alopecias. The tissue deposit is believed to extend the drug’s bioavailability and augment its presence at the follicular level.5 Oral bicalutamide may induce, in some patients, hepatotoxicity, mastalgia, asthenia, myalgia, and decreased libido.2 Mesotherapy has the potential to enhance hair regrowth with fewer side effects for patients with contraindications to the oral form or those seeking improved outcomes.
In our initial investigation, mesotherapy with bicalutamide only improved the hair density in the frontal area in one out of four patients. Adjusting drug concentrations and the frequency of sessions may potentially unveil more favourable outcomes. While pain could pose a challenge to patient compliance, no participants withdrew from our study. The amelioration of seborrhoea stands out as a possible benefit, particularly when FPHL and seborrheic dermatitis co-occur.
Nevertheless, we acknowledge that, although this study is a pilot, the limited sample size and the absence of a control group are constraints in our research.
In conclusion, mesotherapy with bicalutamide presented limited efficacy as a monotherapy for FPHL. The procedure was considered safe despite the pain and minor symptoms reported. Further studies with larger sample sizes controlled with placebo may clarify its safety and efficacy for this condition.
Ethical approval
Ethical approval was not sought for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Female-pattern hair loss: Therapeutic update. An Bras Dermatol. 2023;98:506-19.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know. J Cosmet Dermatol. 2022;21:4171-5.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of oral bicalutamide in female pattern hair loss: A retrospective review of 316 patients. J Am Acad Dermatol. 2020;83:1478-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bicalutamide: A potential new oral antiandrogenic drug for female pattern hair loss. J Am Acad Dermatol. 2020;83:e355-e356.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of mesotherapy: A novel avenue for the treatment of hair loss. J Dermatolog Treat. 2023;34:2245084.
- [CrossRef] [PubMed] [Google Scholar]
- Mesotherapy with dutasteride for androgenetic alopecia: A retrospective study in real clinical practice. J Drugs Dermatol. 2022;21:742-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mesotherapy with bicalutamide: A new treatment for androgenetic alopecia. Int J Trichology. 2023;15:39-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






