Translate this page into:
Metabolomic biomarkers and altered phenylalanine metabolic pathway in preschool children with atopic dermatitis – A pilot study
Corresponding author: Dr. Ying Xiong, Department of Dermatology, Shenzhen Maternity & Child Healthcare Hospital, Shenzhen, China. xiongy2004@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yu J, Chen T, Zhou H, Li S, Wu B, Xiong Y. Metabolomic biomarkers and altered phenylalanine metabolic pathway in preschool children with atopic dermatitis – A pilot study. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1125_2023
Abstract
Background
Atopic dermatitis (AD) has high prevalence in children. Current AD diagnosis and management focuses only on clinical phenotypes, but do not explore the endophenotypes, which are more important because they are a series of biomarkers linking clinical phenotype and genotype
Aims
Metabolomics can qualitatively and quantitatively capture real-time dynamic changes in a wide range of small molecule metabolites. This pilot study evaluated metabolomics biomarkers and altered metabolic pathways in preschool children with AD, aiming to explore the underlying molecular mechanisms and signalling pathways of the disease.
Methods
Blood samples of 23 preschool children with AD and 23 healthy children without AD or any other skin disease were collected. The untargeted metabolomic measurements were performed on a SCIEX-AD ultraperformance liquid chromatography system coupled with an AB SCIEX X500B QTOF system. Characteristics of small molecules in AD children were assessed and their associations with AD clinical index were evaluated. Altered metabolic pathways in AD children were also analysed using a comprehensive metabolomics platform.
Results
A total of 1,969 metabolites were identified, of which AD children exhibited 377 significantly altered metabolites. Multivariate statistical analysis demonstrated that the AD group and the control group could be clearly separated. Volcano plot analysis illustrated that 144 metabolites were up-regulated and 233 metabolites were down-regulated in AD children. The Severity Scoring of Atopic Dermatitis (SCORAD index) showed a moderate-to-strong association with estrogens, carotenes, leukotrienes, flavonols and keto acids in AD children (|r|=0.440–0.557). Several pathways, including the phenylalanine metabolism, were identified as altered in AD children.
Limitations
A small group of children was included in the study; the results need to be validated in larger sample sizes.
Conclusion
Results of this study illustrate potential alterations in metabolites and the phenylalanine metabolic pathway in preschool children with AD. Although this is a pilot study with a limited sample size, it may provide a new perspective for exploring the pathogenesis of AD, and for personalised treatment modalities.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, pruritic and inflammatory skin disease, often associated with other allergic conditions like food allergies, allergic asthma and allergic rhinitis (AR), etc. It is now regarded as a systemic disease. Globally, there are at least 230 million people with AD. In developed countries, up to 10–20% of children are affected. Clinical epidemiological surveys indicate that in 12 major Chinese cities, the prevalence of AD in children aged 1–7 years was around 13% and for those aged 1–12 months, it was as high as 30% in 2014.1,2
Endophenotypes are a series of biomarkers linking clinical phenotype and genotype. A study of these endophenotypes is a new concept proposed in recent years, for research of complex disease. Subtyping heterogeneous diseases based on biomarkers and endophenotypes may lay the groundwork for individualised disease prevention and treatment strategies. The current clinical diagnosis of AD is based on morphological features of skin lesions, distributions of clinical signs and history of atopy. However, AD demonstrates obvious heterogeneities in terms of age, race, geographic location and atopic disease history which may be related to the endophenotype of AD.3 The highly heterogeneous endophenotype of AD results from a complex interaction of intrinsic factors (genetic factors), extrinsic factors (environmental stimulus), skin functions abnormalities (barrier function impairments, epidermal lipids, protease abnormalities, etc.), immune function impairments and microbiota dysbiosis.4
Currently, there is no cure for AD. The primary aim of management is symptom relief, prevention of exacerbation and long-term control. However, it has failed to fully consider the differences between clinical phenotypes and endophenotypes. Given the clinical variability of AD, more sensitive molecular tools or biomarkers are needed to target different endophenotypes, develop individualised strategies and evaluate treatment outcomes.
Metabolomics, an emerging high-throughput omics technology, assists in identifying disease endophenotypes. It can qualitatively and quantitatively capture real-time dynamic changes in a wide range of small molecule metabolites. These fluctuations provide insights into the pathophysiological profile influenced by environmental exposure, genetics and epigenetics.5,6 In current treatment guidelines in most countries, treatment methods for severe AD and mild-to-moderate AD are different. In general, mild-to-moderate patients are given moisturisers and topical anti-inflammatory drugs, while severe patients are treated with a combination of immunosuppressants and cytokine monoclonal antibodies in addition to topical therapy. Variation in severity of disease may also be due to the heterogeneous endophenotype. Therefore, in this pilot study, we aim to explore changes in endogenous metabolites in preschool children with mild-to-moderate AD, which could influence clinical severity.
Methods
Study design
This is a cross-sectional pilot study. The primary objective is to explore the feasibility of identifying possible metabolite changes in preschool children with mild-to-moderate AD. The secondary objective is to explore the potentially altered metabolic pathways. This study was carried out during September and October 2021. A written consent form was obtained from the participating children’s parents before they were recruited. This study was approved by the IRB of Shenzhen Maternity & Child Healthcare Hospital (IRB approval number: SFYLS[2023]012).
Study population
Eligible preschool children with AD (AD group) were recruited with the following inclusion criteria: 1) aged 3–6 years old; 2) diagnosed as AD by the 2020 edition of the Chinese Guidelines for the Diagnosis and Treatment of Atopic Dermatitis in Children; and 3) diagnosed as mild-to-moderate AD with the Severity Scoring of Atopic Dermatitis (SCORAD index) ≤ 50. Totally, 31 eligible children with AD were assessed, but 8 of them were further excluded due to failure of blood sample collection and incomplete data. Twenty-three children with AD remained and were recruited in this pilot study. To match with the AD group, 23 healthy children aged 3–6 years without AD or any other skin or inflammatory disease were also recruited in this pilot study as the control group. Children were excluded if they had 1) inflammatory diseases other than AD; 2) malignant tumours; 3) chronic systemic diseases, such as diabetes, hypothyroidism, etc. 4) autoimmune diseases, such as alopecia areata, vitiligo, urticarial, etc.; 5) acute or chronic infections and 6) undergone phototherapy, systemic therapy with antibiotics or antifungals, systemic anti-inflammatory therapy or immunomodulatory therapy within 4 weeks. Figure 1 illustrates the CONSORT flow diagram.
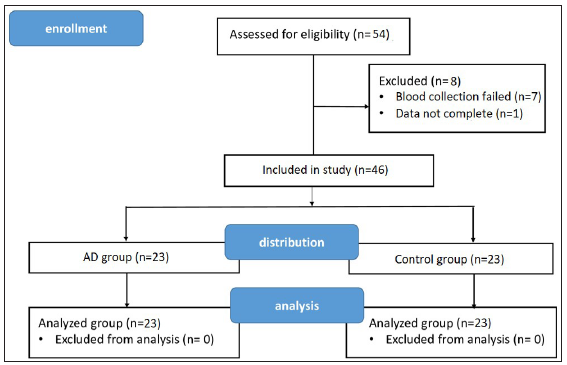
- CONSORT flow diagram.
Sample collection, preparation and LC-MS/MS analysis
The blood sample was taken early in the morning before breakfast using an Ethylene diamine tetraacetic acid (EDTA) anticoagulant tube to prevent clotting. The sample was left at room temperature for a short time, then centrifuged to separate the plasma. This plasma was then stored in a freezer. For testing, the plasma was deproteinised and centrifuged again to prepare it for a detailed analysis using liquid chromatography with tandem mass spectrometry (Liquid chromatography (LC)-Mass spectrophotometer (MS/MS)). A small amount from each sample was also mixed together for quality control to make sure the tests are accurate. Untargeted measurements were carried out using an ultra-performance liquid chromatography (UPLC) system coupled with a tandem mass spectrometry system with a 5 μL injection volume. The positive ion mode and negative ion mode were separated. The flow rate, column temperature and injection volume were carefully controlled. Data were collected in a full scan mode covering a wide range of mass-to-charge ratios (m/z 30−1200). Various parameters like ion spray voltage, gas pressures and heater temperature were set for optimal performance. For identifying metabolites, data were compared against four databases (GNPS, MassBank, HMDB and NIST) using the msSLASH algorithm.7,8 Metabolites with a similarity score above 0.7 were selected for further analysis.
Statistical analysis
All quantitative data were presented as mean ± standard deviation. All categorical data were expressed as percentage. All metabolites were performed with a Shapiro–Wilk test to check the normality of the data. Between-group differences for quantitative demographics and metabolites’ characteristics were compared using an independent t-test or a Wilcoxon Mann−Whitney test, depending on the normality of the data. Between-group differences for categorical demographics were compared using a Chi-square test. Partial least squares discriminant analysis (PLS-DA) was applied as the multivariate statistical analysis to investigate the change of metabolites in the AD group. Volcano plot analysis was also performed. Correlation analyses between the SCORAD index and metabolites levels were performed using Pearson correlation. For avoiding type I error, false discovery rate (FDR) adjustment using the Benjamini–Hochberg procedure was performed in the Python statsmodels module. Statistical significance was accepted at p<0.050. All statistical analyses were performed using IBM Statistical Package for Social Sciences (SPSS Version 26; IBM, Chicago, IL, USA).
Results
Demographics and clinical information
The average age of the AD group was 56.35 ± 5.65 months with 13 boys and 10 girls. The average age of the control group was 55.83 ± 5.84 months with 12 boys and 11 girls. The two groups had no difference in age and gender. The SCORAD index of the AD group ranged from 9 to 36.4 with an average of 21.26 ± 7.58 [Table 1].
| Control Group (n=23) | AD Group (n=23) | p-value | |
|---|---|---|---|
| Age, month | 55.83 ± 5.84 | 56.35 ± 5.65 | 0.760 |
| Gender (female) | 48% | 44% | 0.767 |
| SCORAD index | - | 21.26 ± 7.58 | - |
Untargeted metabolomic analysis
The untargeted analysis totally identified 1,969 different metabolites, out of which 1,591 metabolites were identified in positive ion mode, while 378 metabolites were identified in negative ion mode. The statistical analysis resulted in 377 statistically differing metabolites between the AD group and the control group, out of which 295 were in positive ion mode and 82 were in negative ion mode. Among these statistically differing metabolites, 217 metabolites were further matched in the human metabolome database (HMDB). Figure 2 illustrates 20 metabolites with the largest between-group effect sizes. Multivariate statistical analysis was performed for all metabolites acquired from both positive ion mode and negative ion mode to investigate the change of metabolites in the AD group. On the basis of the PLS-DA model, the AD group and the control group were clearly separated [Figure 3]. Volcano plot analysis was also performed. Among the 377 significantly dysregulated metabolites, 144 metabolites were significantly upregulated, while 233 metabolites were significantly down-regulated in the AD group [Figure 4].
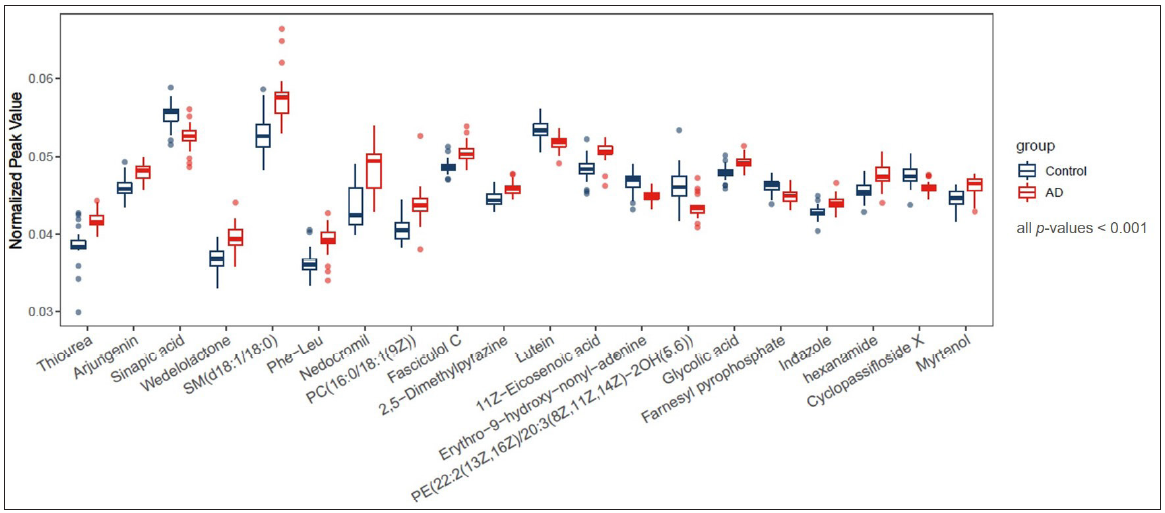
- Twenty metabolites with the largest effect sizes between the AD group and the control group, all p-values < 0.001
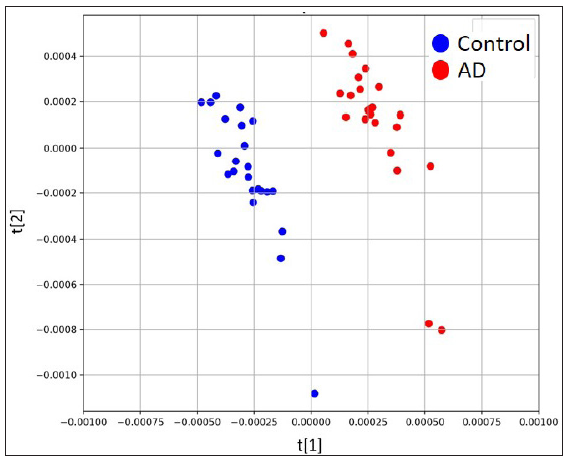
- Scores plots of PLS-DA model based on positive ion mode.
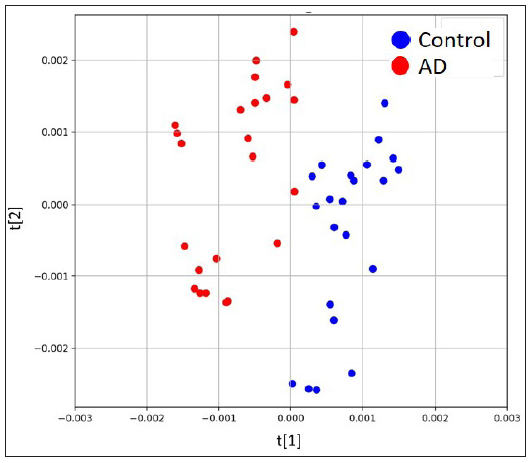
- Scores plots of PLS-DA model based on negative ion mode.
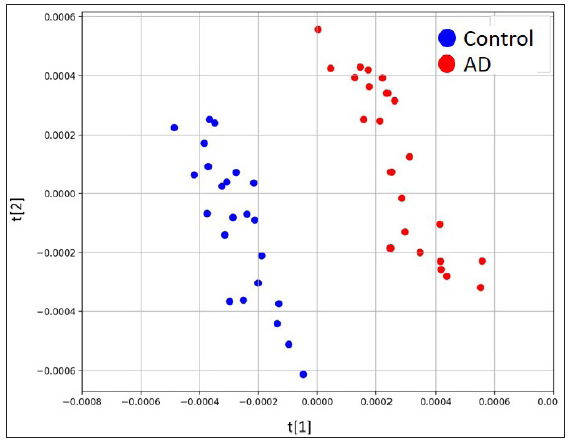
- Scores plots of PLS-DA model based on combination of both modes.
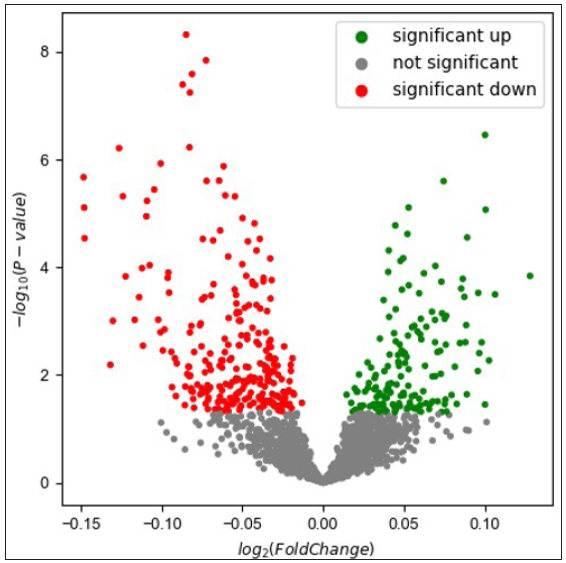
- Volcano plot based on combination of both positive ion and negative ion modes.
Associations between SCORAD index and metabolites in preschool children with AD
Associations between the SCORAD index and metabolites levels in the AD group were evaluated. For the abovementioned 377 significantly dysregulated metabolites in the AD group, Pearson correlation coefficient was calculated between each metabolite and the SCORAD index. Results demonstrated that the SCORAD index was moderately-to-strongly associated with several types of metabolites, including estrogens, carotenes, leukotrienes, flavonols, keto acids, etc. Figure 5 illustrates examples of estriol (r = −0.546, p = 0.010); meso-zeaxanthin: one type of carotene (r = −0.557, p=0.009); leukotriene B5: an anti-inflammatory agent being demonstrated associating with allergic reactions (r = −0.440, p = 0.046), morin: one type of pentahydroxyflavone, an antioxidant and anti-inflammatory agent (r = −0.442, p=0.045); and phenylpyruvic acid (r = −0.446, p=0.043). However, after performing FDR adjustment, the p-values became ≥ 0.050.
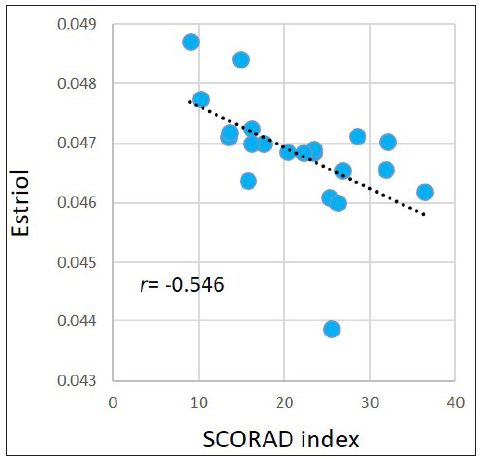
- Pearson correlations between the SCORAD index and estriol in the AD group.
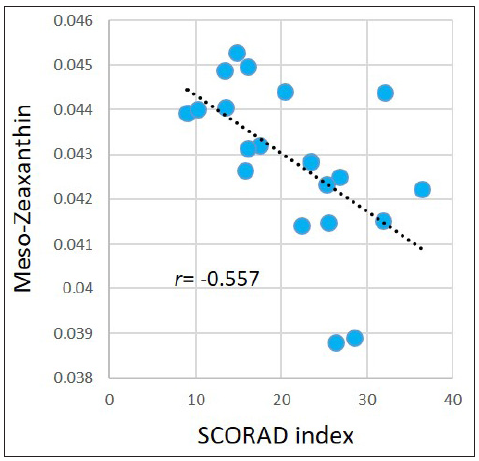
- Pearson correlations between the SCORAD index and meso-Zeaxanthin in the AD group.
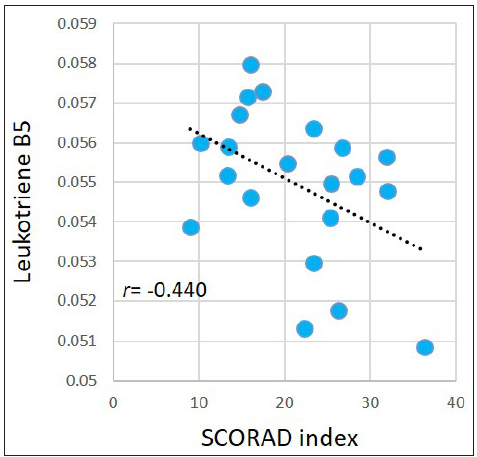
- Pearson correlations between the SCORAD index and leukotriene B5 in the AD group.
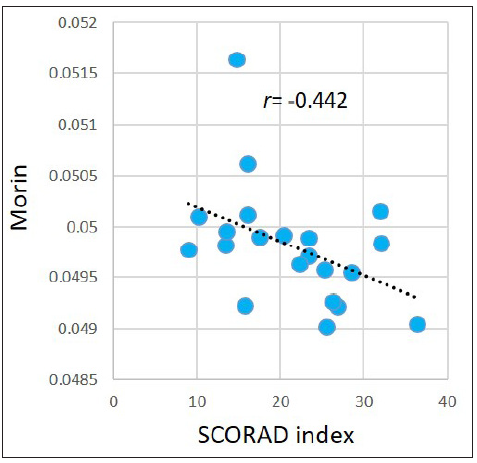
- Pearson correlations between the SCORAD index and morin in the AD group.
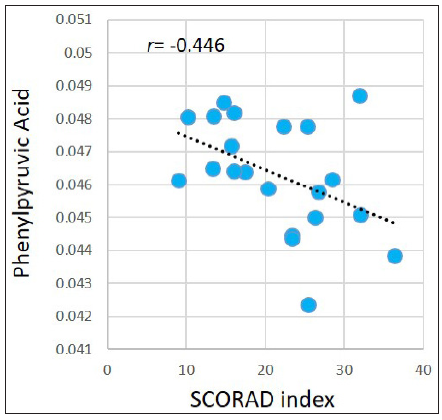
- Pearson correlations between the SCORAD index and phenylpyruvic acid in the AD group.
Altered metabolic pathways in preschool children with AD
Finally, the altered metabolites were fed into Metabo Analyst 5.0, a comprehensive platform dedicated for metabolomics data analysis, for analysing the altered metabolic pathway in the AD group. Several pathways, including the phenylalanine metabolism (p = 0.013, FDR = 0.544), the steroid hormone biosynthesis (p = 0.038, FDR = 0.794), the glycerophospholipid metabolism (p = 0.025, FDR = 0.708) and the phenylalanine tyrosine and tryptophan biosynthesis (p = 0.050, FDR = 0.898), were identified as altered in the AD group. As illustrated by Figure 6, in the phenylalanine metabolism, the L-phenylalanine (p = 0.049) and the phenylpyruvate (p = 0.012) were significantly elevated in the AD group, while the phenylacetaldehyde was significantly down-regulated (p = 0.015).
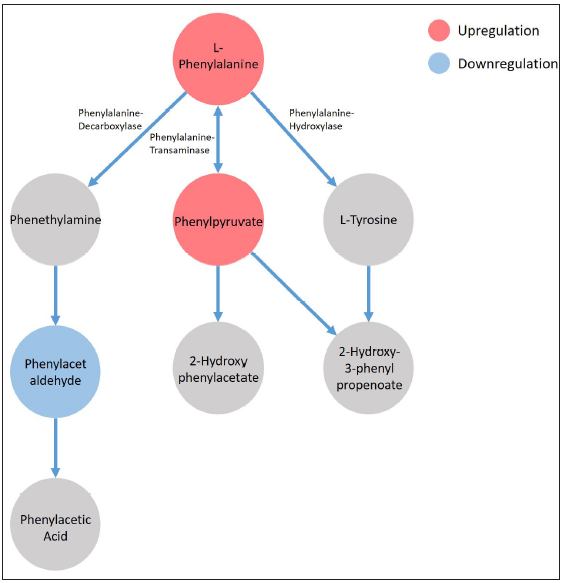
- The phenylalanine metabolism was identified as significantly altered in the AD group by MetaboAnalyst 5.0 platform.
Discussion
AD is a chronic inflammatory skin disease that usually occurs in infants and children. It may experience a chronic relapsing course, lasting from childhood to adolescence and even to adulthood. Persistent AD appears to be significantly associated with early childhood onset and the coexistence of other idiosyncratic reactions. The pathophysiological mechanisms of AD are complex, including genetics, skin barrier functions, cellular immunity and environmental factors. Metabolomics reveals the metabolic signature of genetic variation and environment in disease states at the molecular level. Identifying altered metabolites and metabolic pathways facilitates discovery of novel biomarkers, early diagnosis, and making new treatment strategies for the disease. In this pilot study, we performed untargeted metabolomic analysis using blood samples collected from 23 preschool children with AD and 23 healthy children. We totally identified 1,969 different metabolites. Among them, 217 metabolites were matched in the HMDB while exhibiting significant differences between the AD group and the control group. By analysing metabolic pathways, we identified several pathways, including the phenylalanine metabolism, the phenylalanine tyrosine and tryptophan biosynthesis, the steroid hormone biosynthesis, the linoleic acid metabolism and the glycerophospholipid metabolism, which were altered in the AD group. All of these metabolic pathways have been shown to be closely associated with inflammatory diseases and/or allergic skin disease,9–13 while the phenylalanine metabolism alteration was reported in AD for the first time.
In previous literature, the altered metabolomic profile reported in AD children and AD adults was not exactly the same, and the results showed inconsistency. In a study of children with AD, Huang et al. used untargeted metabolomics and targeted eicosanoids analysis and found that various eicosanoids involved in the lipoxygenase (LOX) and cyclooxygenase (COX) pathways, including leukotriene B4 (LTB4), thromboxane 2 (TXB2), prostaglandins, hydroxyl octadecadienoic acids (HODEs) and most hydroxyeicosatetraenoic acids (HETEs), were significantly increased, while conjugated bile acids were decreased. AD children with high IgE levels were closely related to energy metabolism disorders and amino acid metabolism abnormalities, while AD patients with normal IgE levels have increased cytochrome P450 (CYP) metabolite levels.14 In one study of eicosanoid/docosanoid in AD adult patients, researchers found significant reductions in lipoxin A4 (LXA4), leukotriene B5 (LTB5), docosahexaenoic acid (DHA) and maresin (MAR). The untargeted metabolomic results showed that COX1 and ALOX12B expression, as well as COX and 12/15-LOX metabolites in the serum of AD adult patients were significantly increased.15 Interestingly, another targeted metabolomics study using the same technology platform (HPLC-MS/MS), but different blood samples peripheral blood mononuclear cells (PBMCs), demonstrated the opposite with both COX- and LOX-mediated pathways and their corresponding products (except arachidonic acid) down-regulated in PBMCs in AD patients.16 Other studies also identified significant metabolic alterations in linoleic acid, phosphatidylcholines, acylcarnitines, n-6 fatty acid metabolism and the energy cycle.13,17
Compared to the controls, our results showed that children with AD exhibited an upregulated phenylalanine level (p=0.049). In previous studies, phenylalanine was reported to be elevated in patients with contact dermatitis and infants with food allergies,12,18 which is consistent with our findings. Phenylalanine is an essential amino acid that cannot be synthesised by the human body. Elevated serum levels of these amino acids may be associated with altered catabolism. In recent years, more and more attention has been focussed on the role of phenylalanine in acute inflammatory diseases. Studies revealed that a high serum level of phenylalanine is associated with sustained immune activation,19 serious infections,20,21 and high mortality in patients with heart failure.22 This study demonstrated that the phenylalanine metabolism was significantly associated with AD situation which is consistent with previous literature reporting that AD is closely related to abnormal amino acid metabolism, altered energy circulation and systemic inflammation.14
Besides upregulated phenylalanine level, we also discovered upregulated phenylpyruvate level in the AD group (p=0.012). This finding is consistent with previous studies showing that elevated phenylalanine stimulates transaminases, leading to increased alternative pathway that produces phenylpyruvate and finally resulting in phenylpyruvate up-regulation.9 In addition, our results demonstrated that the serum level of phenylacetaldehyde, a secondary metabolite of phenylalanine, was down-regulated in children with AD. This phenomenon suggested that AD may affect the activity of phenylalanine decarboxylase and may further inhibit the alternative pathway of ‘phenylalanine–phenethylamine–phenylacetaldehyde’. So far, this hypothesis has not been reported. Phenylalanine is an essential amino acid for neurotransmitter biosynthesis.23 In the human body, it mostly converts into tyrosine and together with tyrosine to synthesise substantial neurotransmitters and hormones. Phenethylamine is an endogenous natural monoamine alkaloid with stimulatory effects that cause the release of neurotransmitters, including dopamine and serotonin. Phenethylamine binds to amine-related receptors, thereby inhibiting the reuptake of dopamine, serotonin, and norepinephrine.24 AD is characterised by chronic itching. It often manifests as itching during the night-time. After repeated scratching, the release of inflammatory factors increases, aggravating the itching sensation and forming an itching–scratching cycle. AD pruritus involves complex nerve conduction pathways. In the skin, the nerve and immune system interact bidirectionally through neuromediators (neuropeptides and neurotransmitters) released by skin nerves and immune cells.25
Our findings may contribute significantly to understanding the pathogenesis of AD and may open new avenues for disease control and symptom alleviation. By identifying such metabolic alterations, researchers can gain deeper insights into the biochemical pathways involved in AD which can shed light on its underlying causes. In addition, these altered metabolites may serve as biomarkers for AD. This can help in early diagnosis and in monitoring the progression of disease or response to treatment, enabling more timely and targeted interventions. Understanding the role of phenylalanine metabolism in AD may also lead to the exploration of new therapeutic targets as well as personalised treatment approaches. Finally, by targeting the metabolic pathways involved in AD, it might be possible to reduce inflammation and other symptoms associated with the disease, leading to improved quality of life for patients.
Limitations
One major limitation of this pilot study is that we did not calculate the sample size based on effect size anticipation but only enrolled based on available samples. This led to a small sample size with only 23 AD samples which could have affected the accuracy and reliability of the results. In future studies, we need to confirm our findings using the results of this study with a pre-calculated sample size. We also need to perform targeted metabolomic analysis with larger sample sizes. In addition, we will apply machine learning techniques to identify AD metabolic biomarkers, promoting preventive strategies, early diagnosis, and personalised treatments in young children with AD.
Conclusion
In summary, alterations in the phenylalanine metabolism may promote the occurrence and development of AD. Based on metabolomics, our results elucidate potential alterations in metabolites and the phenylalanine metabolic pathway in preschool children with AD. This pilot study provided a new perspective for exploring the pathogenesis of AD, as well as new references for personalised treatments. Future studies of targeted metabolomic analysis with larger sample sizes combining with machine learning techniques are warranted to confirm these findings and explore related mechanisms.
Ethical approval
This study was approved by the IRB of Shenzhen Maternity & Child Healthcare Hospital (IRB approval number: SFYLS[2023]012).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
We acknowledge the support from the Clinical Research Cultivation Project of The First Affiliated Hospital of Nanchang University: YFYLCYJPY202003 and the Major Science and Technology R&D Project of the Science and Technology Department of Jiangxi Province: 20213AAG01013.
Conflicts of interest
He Zhou who has conducted the test is an industrial author and his inclusion has no bearing on the study outcome.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Prevalence of atopic dermatitis in Chinese children aged 1–7 ys. Sci Rep. 2016;6:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phenotypic analysis of atopic dermatitis in children aged 1–12 months: Elaboration of novel diagnostic criteria for infants in China and estimation of prevalence. J Eur Acad Dermatol Venereol. 2019;33:1569-76.
- [CrossRef] [PubMed] [Google Scholar]
- Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-55.
- [CrossRef] [PubMed] [Google Scholar]
- Leveraging multilayered “Omics” data for atopic dermatitis: A road map to precision medicine. Front Immunol. 2018;9:2727.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The metabolomics of childhood atopic diseases: A comprehensive pathway-specific review. Metabolites. 2020;10:511.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exploratory metabolomics of metabolic syndrome: A status report. World J Diabetes. 2019;10:23-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A fast and memory‐efficient spectral library search algorithm using locality‐sensitive hashing. Proteomics. 2020;20:2000002.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- GlycoSLASH: Concurrent glycopeptide identification from multiple related LC-MS/MS data sets by using spectral clustering and library searching. J Proteome Res. 2023;22:1501-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phenylketonuria: Metabolic alterations induced by phenylalanine and phenylpyruvate. Am J Clin Nutr. 1975;28:183-8.
- [CrossRef] [PubMed] [Google Scholar]
- Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: A pilot study. Eur J Nutr. 2020;59:2119-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Revealing the correlation between altered skin lipids composition and skin disorders. Cosmetics. 2021;8:88.
- [CrossRef] [Google Scholar]
- Activation of tryptophan and phenylalanine catabolism in the remission phase of allergic contact dermatitis: A pilot study. Int Arch Allergy Immunol. 2016;170:262-8.
- [CrossRef] [PubMed] [Google Scholar]
- Linoleic acid metabolite levels and transepidermal water loss in children with atopic dermatitis. Ann Allergy Asthma Immunol. 2008;100:66-73.
- [CrossRef] [PubMed] [Google Scholar]
- Serum metabolomics study and eicosanoid analysis of childhood atopic dermatitis based on liquid chromatography–mass spectrometry. J Proteome Res. 2014;13:5715-23.
- [CrossRef] [PubMed] [Google Scholar]
- Transcriptomic and lipidomic profiling of eicosanoid/docosanoid signalling in affected and non‐affected skin of human atopic dermatitis patients. Exp Dermatol. 2019;28:177-89.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced lipoxygenase and cyclooxygenase mediated signaling in PBMC of atopic dermatitis patients. Prostaglandins Other Lipid Mediat. 2013;107:35-42.
- [CrossRef] [PubMed] [Google Scholar]
- Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation. PLoS One. 2017;12:e0188580.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Plasma metabolomic profiles associated with infant food allergy with further consideration of other early life factors. Prostaglandins Leukot Essent Fatty Acids. 2020;159:102099.
- [CrossRef] [PubMed] [Google Scholar]
- Immune activation in patients with Alzheimer’s disease is associated with high serum phenylalanine concentrations. J Neurol Sci. 2013;329:29-33.
- [CrossRef] [PubMed] [Google Scholar]
- Increased mortality of acute respiratory distress syndrome was associated with high levels of plasma phenylalanine. Respir Res. 2020;21:1-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phenylalanine-and leucine-defined metabolic types identify high mortality risk in patients with severe infection. Int J Infect Dis. 2019;85:143-9.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail. 2020;7:2884-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Amino acids and immune response: A role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. 2015;21:9-17.
- [CrossRef] [PubMed] [Google Scholar]
- 2-Phenylethylamine (PEA) ameliorates corticosterone-induced depression-like phenotype via the BDNF/TrkB/CREB signaling pathway. Int J Mol Sci. 2020;21:9103.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neuroimmunological mechanism of pruritus in atopic dermatitis focused on the role of serotonin. Biomol Ther (Seoul). 2012;20:506.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





