Translate this page into:
Metastatic anaplastic large cell lymphoma of the omentum presenting as an ulcerated nodule on the back
Corresponding author: Dr. Jimena Agostina Miranda, Department of Dermatology, Hospital Nacional de Clínicas, Santa Rosa 1564, X5000 ETF, Córdoba, Argentina, jam.dermatologia@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Miranda JA, Elías MB, Mazzotta MM, Zalazar ÉV. Metastatic anaplastic large cell lymphoma of the omentum presenting as an ulcerated nodule on the back. Indian J Dermatol Venereol Leprol 2023;89:106-9.
Sir,
Anaplastic lymphoma kinase (ALK)-negative anaplastic large-cell lymphoma is a rare peripheral T-cell non-Hodgkin lymphoma that belongs to the group of CD30-positive lymphoproliferative disorders and tends to affect adults over the age of 40 with a bad prognosis.1-3 It involves the lymph nodes and extranodal sites, but the involvement of the omentum as the primary site is rare. In ALK-negative anaplastic large-cell lymphoma patients, skin is one of the most common sites of spread. Secondary cutaneous lesions may be difficult to differentiate from primary cutaneous anaplastic large cell lymphoma.1,4
Herein, we describe an unusual case of a 67-year-old woman who presented with an ulcerated lesion on the back as secondary cutaneous involvement of primary ALK-negative anaplastic large-cell lymphoma of the omentum.
A 67-year-old woman presented with a two-week history of a rapidly growing painful ulcerated lesion on her back. She also complained of sporadic abdominal pain and weight loss for four months. Physical examination revealed ulcerated violaceous nodule measuring 3 cm in diameter in the lumbar region [Figure 1a]. Ultrasound of soft tissue showed a nodular mass with cystic-necrotic areas. Differential diagnosis of tuberculosis and neoplasia were initially considered. The lesion was biopsied for microbiological culture and histological examination. Bacterial, mycobacterial, and fungal cultures were negative. Haematoxylin and eosin staining showed dermal perivascular infiltration of large atypical lymphoid cells extending into the subcutaneous fat [Figures 1b and c ]. Immunohistochemistry was positive for CD3, CD4, CD7, CD8, CD30 (uniform and strong) [Figure 1d], and negative for CD5, CD56, epithelial membrane antigen, Epstein-Barr virus and ALK-1 [Figure 1e]. The expression of Ki-67 was 80% positive. The diagnosis of primary systemic anaplastic large cell lymphoma with skin involvement was considered. A computed tomography scan of the abdomen and pelvis revealed a poorly defined mass with soft tissue density in the omentum, in contact with the transverse colon and free fluid in the cul-de-sac [Figure 2a]. Laboratory workup was notable for an erythrocyte sedimentation rate of 124 mm/h and C-reactive protein of 14.9 mg/dL. Blood routine examination showed white blood cell count at 19,500/μL, haemoglobin count at 10.4 g/dL and platelet count at 745,000/μL. Serologic tests for HIV, hepatitis C, hepatitis B, human T-lymphotropic virus type 1 (HTLV-I) and Epstein-Barr virus were negative. She underwent an exploratory laparotomy for resection of the abdominal mass. Histology with immunohistochemical staining showed the same features as the previous skin biopsy [Figures 2b to d]. The diagnosis of primary ALK-negative anaplastic large cell lymphoma of the omentum with cutaneous metastases was made. The bone marrow biopsy result was normal. Although she was treated with four cycles of cyclophosphamide, adriamycin, vincristine, and prednisone regimen, her clinical condition worsened with decreasing mental status. Magnetic resonance imaging of the brain revealed multiple brain metastases. She died three months later due to disease progression.

- A round ulcerated violaceous nodule in the lumbar region
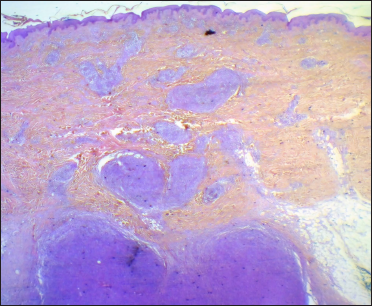
- Dense dermal and hypodermal infiltrate of large atypical lymphocytes (haematoxylin and eosin, ×4)
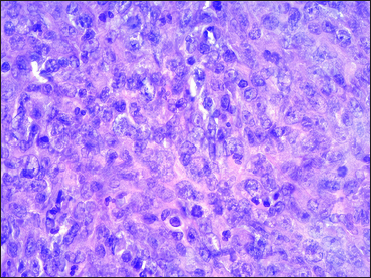
- Large pleomorphic hyperchromatic lymphoid cells with prominent nucleoli (haematoxylin and eosin, ×400)
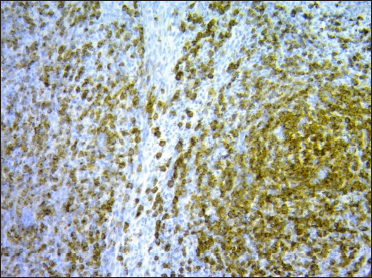
- The atypical lymphocytes stained positive for CD30 (×100)
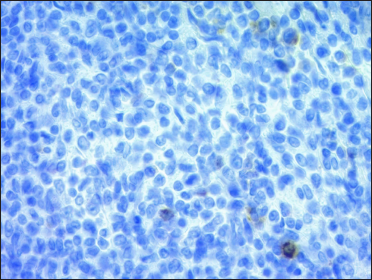
- The atypical lymphocytes stained negative for ALK (×400)
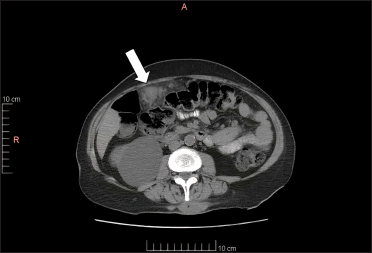
- Abdominal CT image shows a tumour in the omentum, in contact with the transverse colon (white arrow)
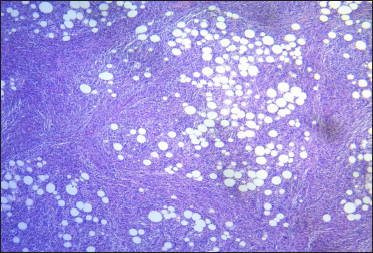
- Omentum: adipose tissue with dense and diffuse infiltrate of large atypical lymphoid cells (haematoxylin and eosin, ×10)
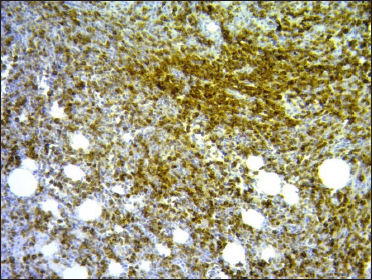
- Omentum: CD30-positive immunostaining (×100)
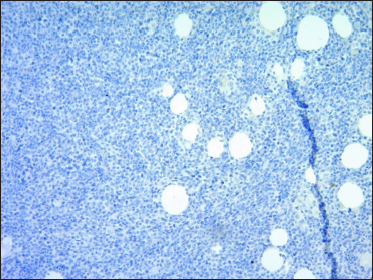
- Omentum: ALK-negative immunostaining (×50)
ALK-negative anaplastic large cell lymphoma is an aggressive CD30-positive T-cell neoplasm morphologically indistinguishable from its ALK-positive counterpart.1 ALK-positive refers to the presence of a protein called ALK. These tumours have typically favourable outcomes. In contrast, ALK-negative anaplastic large-cell lymphoma is characterized by the lack of expression of ALK and this failure to express ALK protein has been shown to portend a worse prognosis.2,3
The exact prevalence of this tumour is unknown. It tends to affect patients over 40 years, unlike ALK-positive anaplastic large-cell lymphoma that occurs in children and young adults. A male predominance with a male:female ratio of 1.5:1 is reported.4
ALK-negative anaplastic large-cell lymphoma primarily involves peripheral, mediastinal or abdominal lymph nodes, but the disease can present at any site, including the central nervous system, oral cavity, bladder, pancreas and liver.1,5 However, to the best of our knowledge primary involvement of the omentum has not been reported.
In ALK-negative anaplastic large-cell lymphoma patients, the skin, liver and lung are the most common sites for secondary spread, compared with soft tissue and bone in ALK-positive anaplastic large-cell lymphoma.1 Of note, primary cutaneous anaplastic large cell lymphoma is typically negative for ALK and can be clinically indistinguishable from secondary skin involvement by systemic ALK-negative anaplastic large-cell lymphoma. However, this subtype rarely disseminates.6
It is important to differentiate cases of systemic ALK-negative anaplastic large-cell lymphoma from ALK-negative primary cutaneous anaplastic large-cell lymphoma, as the prognosis varies greatly. The primary cutaneous anaplastic large-cell lymphoma has a very favourable 5-year overall survival of 90% as compared to 49% for systemic ALK-negative anaplastic large-cell lymphoma.3
Cutaneous clinical presentation in systemic ALK-negative anaplastic large-cell lymphoma includes nodules, plaques, and ulcerated lesions without a predominant localization. Rieger et al., reported a case of an ALK-negative systemic anaplastic large-cell lymphoma presenting as a violaceous plaque on the scalp of a 79-year-old man.3 Long et al., described a 47-year-old man with anaplastic large-cell lymphoma cutaneous metastases presenting as multiple light pink annular papules and nodules with central ulceration on the face and neck.7
Yang et al., reported one case of systemic ALK-negative anaplastic large-cell lymphoma with secondary ulcerated cutaneous lesions and an aggressive progression although treated by cyclophosphamide, adriamycin, vincristine and prednisone.8 Alcalá et al., confirmed the diagnosis of breast implant-associated ALK-negative anaplastic large-cell lymphoma in old women with breast cutaneous lesions, treated by cyclophosphamide, adriamycin, vincristine, and prednisone chemotherapy without response.9
In our case, we excluded the diagnosis of primary cutaneous anaplastic large-cell lymphoma with metastasis to the omentum since the patient presented with skin lesion four months after the onset of abdominal pain. In addition, the disease progressed aggressively with metastases to other organs leading to her death in a few months.
Biopsies are challenging as histological findings can emulate other hematologic and solid malignancies.10 Immunohistochemical analysis is necessary for definitive diagnosis. Tumour cells can express diverse surface receptors including CD3, CD4, CD7, CD8, CD43, CD56 and EMA, but a diffuse and strong positive staining for CD30 is characteristic. ALK immunohistochemistry is negative by definition.11 In the present case, the tumour showed diffuse expression of CD30, CD3, CD4, CD7 and CD8, and negative for ALK stain, thus concluding the diagnosis of ALK-negative anaplastic large-cell lymphoma.
Chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone) is the first-line treatment of ALK-negative anaplastic large-cell lymphoma. It may be followed by autologous stem cell transplantation after the first complete remission.12 However, recurrences are frequent.1 Although our patient underwent treatment with cyclophosphamide, adriamycin, vincristine, and prednisone regimen, the disease rapidly progressed.
In conclusion, this case emphasises the involvement of skin as a visible and diagnostic sign of systemic neoplasia. Moreover, in this clinical presentation, it is important to differentiate primary from secondary ALK-negative anaplastic large-cell lymphoma, as the prognosis can vary significantly. Diagnosis of such cases represents a challenge and may be based upon a combination of clinical behaviour, pathological morphology and immunophenotype.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Patient’s consent is not required as the patient’s identity is not disclosed or compromised.
References
- Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol. 2013;85:206-15.
- [CrossRef] [PubMed] [Google Scholar]
- Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40:36-43.
- [CrossRef] [PubMed] [Google Scholar]
- ALK-negative systemic intravascular anaplastic large cell lymphoma presenting in the skin. J Cutan Pathol. 2011;38:216-20.
- [CrossRef] [PubMed] [Google Scholar]
- Anaplastic large cell lymphomas: ALK positive, ALK negative, and primary cutaneous. Adv Anat Pathol. 2015;22:29-49.
- [CrossRef] [PubMed] [Google Scholar]
- ALK Negative Anaplastic Large Cell Lymphoma. 2021. Treasure Island (FL): StatPearls Publishing; StatPearls [Internet] [cited 2021 Nov 10]. Available from:
- [Google Scholar]
- The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: Comparison with anaplastic large-cell lymphoma of nodal origin. Blood. 1996;87:3437-41.
- [PubMed] [Google Scholar]
- Recurrent systemic anaplastic large cell lymphoma: Rapid onset and resolution of cutaneous metastases. JAAD Case Rep. 2020;6:124-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous anaplastic large-cell lymphoma should be evaluated for systemic involvement regardless of ALK-1 status: Case reports and review of literature. Am J Clin Dermatol. 2011;12:203-9.
- [CrossRef] [PubMed] [Google Scholar]
- Skin involvement as the first manifestation of breast implant-associated anaplastic large cell lymphoma. J Cutan Pathol. 2016;43:602-608.
- [CrossRef] [PubMed] [Google Scholar]
- Anaplastic large cell lymphoma: One or more entities among T-cell lymphoma? Hematol Oncol. 2009;27:161-70.
- [CrossRef] [PubMed] [Google Scholar]
- ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496-504.
- [CrossRef] [PubMed] [Google Scholar]
- The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17-25.
- [CrossRef] [PubMed] [Google Scholar]





