Translate this page into:
Methicillin-resistant Staphylococcus aureus colonization and disease severity in atopic dermatitis: A cross-sectional study from South India
2 Department of Dermatology and Venereology, Government Medical College, Kottayam, Kerala, India
3 Department of Community Medicine, Government Medical College, Kottayam, Kerala, India
Correspondence Address:
George Kurien
Department of Dermatology and Venereology, Government Medical College, Kottayam, Kerala
India
| How to cite this article: Jagadeesan S, Kurien G, Divakaran MV, Sadanandan SM, Sobhanakumari K, Sarin A. Methicillin-resistant Staphylococcus aureus colonization and disease severity in atopic dermatitis: A cross-sectional study from South India. Indian J Dermatol Venereol Leprol 2014;80:229-234 |
Abstract
Background: Colonization by methicillin-resistant Staphylococcus aureus (MRSA) in atopic dermatitis is little studied but has therapeutic implications. It may have a role in disease severity given the additional virulence factors associated. Aims: Our aims were to record the proportion of patients with MRSA colonization in atopic dermatitis and to ascertain if any association exists between MRSA colonization and disease severity. Methods: An observational cross-sectional study involving children aged≤12 years with atopic dermatitis attending the outpatient department of Government Medical College, Kottayam was conducted. Socio-demographic data, exacerbating factors and risk factors for hospital care-associated MRSA were documented. Extent of atopic dermatitis was recorded using a standardized scale (Eczema Area Severity Index, EASI). Skin swabs were taken from anterior nares and the worst affected atopic dermatitis sites for culture and sensitivity. Results: Of the 119 subjects recruited during the study period (November 2009-April 2011), Staphylococcus aureus was isolated from 110 (92.4%) patients and MRSA from 30 (25.21%) patients. A total of 18 patients with MRSA had risk factors for healthcare associated-MRSA. The patients whose cultures grew MRSA were found to have significantly higher EASI score when compared to those patients colonized with methicillin sensitive Staphylococcus aureus (P < 0.01). Presence of Staphylococcus aureus, early age of onset, presence of food allergies, seasonal exacerbation and inadequate breastfeeding did not seem to influence disease severity. Conclusions: There is a high degree of prevalence of MRSA (25.2%) in atopic dermatitis and presence of MRSA is associated with increased disease severity. Further studies are needed to validate these findings.INTRODUCTION
Atopic dermatitis is a chronic, relapsing inflammatory skin disease. There is increased colonization of the eczematous and/or uninvolved skin of atopic dermatitis patients by Staphylococcus aureus. [1],[2],[3] Reports suggest that the emergence of community acquired-methicillin resistant Staphylococcus aureus strains in the general population could pose new challenges in atopic dermatitis. [4],[5] Nevertheless, limited data is available regarding the proportion of patients colonised with methicillin-resistant Staphylococcus aureus (MRSA) in pediatric atopic dermatitis. With antibiotics being commonly used empirically for treating secondary skin infections in atopic dermatitis, a thorough understanding of the epidemiology of bacterial colonization and superinfection is necessary for formulating appropriate therapeutic interventions. [6] This becomes especially important in a country like India where antibiotic use is widespread and an upsurge in MRSA infections has been reported. [7] There are a few studies in the literature which have estimated the prevalence of MRSA in atopic dermatitis patients, but none have been reported from India. Furthermore, there is a theoretical possibility of increased disease severity with MRSA infections when compared to methicillin susceptible Staphylococcus aureus infections as community acquired-MRSA are known to harbor many toxins such as the Panton Valentine leukocidin, which are absent in methicillin sensitive Staphylococcus aureus. [8] The primary aim of this study was to record the prevalence of MRSA in atopic dermatitis patients attending our outpatient clinics and to determine if there was any association between MRSA colonization and disease severity.
METHODS
After obtaining ethical clearance from the Institutional Review board, an observational cross-sectional study was conducted in the dermatology outpatient department of Government Medical College, Kottayam, which is an 837-bedded tertiary care teaching hospital in Kerala. The study subjects were all consecutive patients below 12 years of age attending our outpatient clinics from November 2009 to April 2011 who were diagnosed as having atopic dermatitis using the Hanifin and Rajka criteria. [9] Informed consent was taken from the parents/guardians after detailed explanation of study procedures. Patients with a history of recent antibiotic use, either systemic or topical, in the past 4 weeks were excluded. A detailed history of symptoms along with socio-demographic data, predisposing and exacerbating factors, dietary history and risk factors for the presence of health care-associated MRSA if any, were documented.
Bacteriologic methods
Qualitative bacterial culture and sensitivities from swabs of the anterior nares and the two most severely affected sites were obtained from all the subjects. All cultures were plated in mannitol salt agar media for the growth of Staphylococcus aureus. On media growing Staphylococcus aureus , methicillin sensitivity tests were performed by the Kirby-Bauer disk diffusion method.
Eczema area severity index
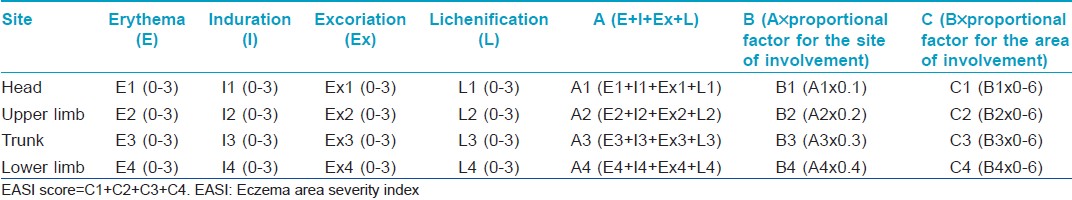
The severity of the disease was recorded by a single observer using EASI, a scoring system described by Tofte S et al., in 1998, as a modification of Psoriasis Area Severity Index (PASI). [10] The EASI is a composite index [Table - 1] including an assessment of disease extent and percent of body surface area involved, converted to a proportional factor (scale of 0-6), in four body regions (head and neck, upper limbs, trunk and lower limbs). The proportions allocated to each body region in patients above 8 years are 10% for head and neck, 20% for upper extremities, 30% for trunk and 40% for lower extremities; whereas in those less than 8 years, the proportions are 20% for head and neck, 20% for upper extremities, 30% for trunk and 30% for lower extremities. The assessment of disease extent is by grading erythema (E), infiltration and ⁄ or papulation (I), excoriation (Ex) and lichenification (L), each on a scale of 0-3. The algorithm for calculating the EASI uses, for each body region, the sum of the clinical sign scores (E + I + Ex + L) multiplied by the area, multiplied by the proportional factor. The total EASI score is the sum of the four body region scores. The EASI has a minimum score of 0 and a maximum of 72. EASI has been validated in several studies and has shown good reliability, consistency and sensitivity to change. [11],[12],[13] For analyzing the severity of disease, those cases with EASI score <6 were classified as having mild disease, those with EASI ≥6 and <18 as moderate and those with EASI ≥18 as having severe disease.
Statistical analysis
Data was analyzed by the software application Statistical Package for the Social Sciences software (SPSS) version 16.0 (IBM, NY). MRSA colonization was defined as cultures growing MRSA from at least one body site. Colonization with methicillin sensitive Staphylococcus aureus (MSSA) was defined as patients harboring MSSA alone, without MRSA isolation from any of the sites. EASI being a composite index, the difference between the mean EASI scores of MRSA and methicillin sensitive Staphylococcus aureus isolated patients were assessed by the non-parametric test, Mann-Whitney U, to ascertain any association with disease severity and the presence of MRSA. Further, patients were also categorized based on the presence/absence of other factors that could influence severity viz. early age of onset, food allergy, seasonal exacerbation, inadequate breast feeding and presence of Staphylococcus aureus and their mean EASI scores compared using the same test. Moreover, EASI score was categorized into mild, moderate and severe and the association of the score with the presence of methicillin resistant or sensitive Staphylococcus aureus was assessed by the Chi-square test. A ′P′ value less than 0.05 was taken as significant in all cases.
RESULTS
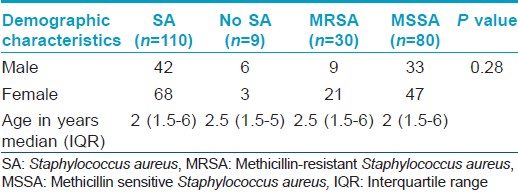
A total of 119 patients were enrolled in the study, all less than 12 years with the youngest patient being 3 months old. The mean age of the subjects was 3.5 years and females dominated with a female:male ratio of 1.47:1. The age and sex distribution of the sample population is presented in [Table - 2]. Most of the children had an early onset of disease with 104 (87.4) patients developing the disease during infancy. The mean age of onset was 1.07 years.
Of the 119 patients, 94 (79.0%) had a personal and/or family history of atopy, of which 30 had a personal history of atopy alone. As far as food allergies were concerned, 66.4% of our children had history of exacerbation of disease following intake of certain food items. Cow′s milk (26.9%) was the most common incriminated food item followed by egg (19.3%). A history of seasonal exacerbation was present in 40.3% patients, most commonly in winters. As many as 20 children within this cohort were living separately from their mothers who were working abroad, of whom 15 were breastfed for less than 4 months.
The disease severity in all cases was recorded by the EASI scoring system. [10] The majority, i.e., 72 (60.5%) patients, had moderate disease, whereas 28 (23.3%) had mild disease, and 19 (16.0%) had severe disease. The lowest score obtained was 3.6 and the highest, 46.4 (median 8.3, interquartile range 4.5).
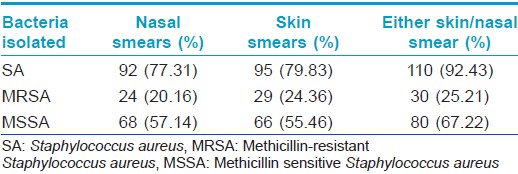
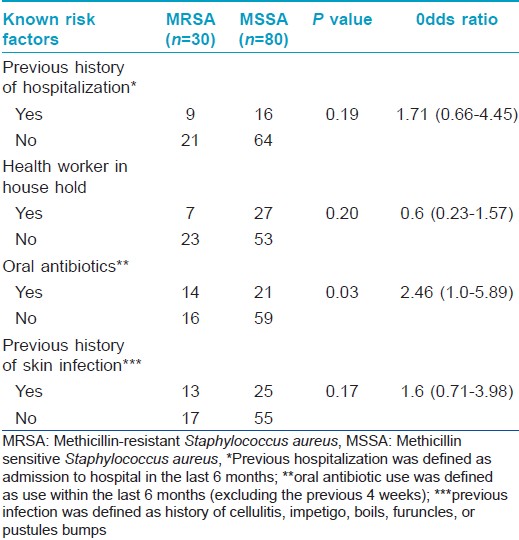
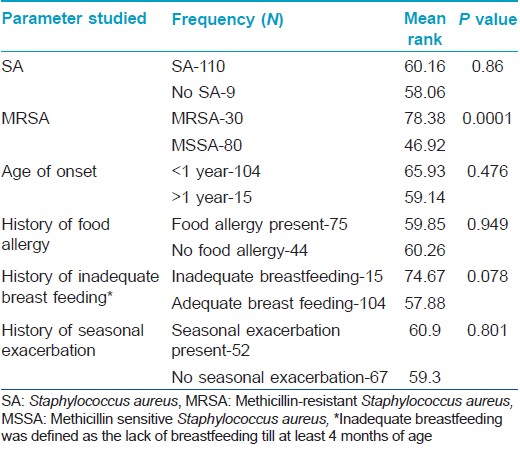
Staphylococcus aureus was isolated from the anterior nares in 92 (77.3%) and skin lesions in 95 (79.8%) subjects, with 110 patients (92.4%) having Staphylococcus aureus positivity from at least one site. MRSA was isolated from 24 (20.2%) nasal smears and 29 (24.4%) skin smears. Total prevalence of MRSA in the study population was found to be 25.2% (30 out of 119 patients, 27.3% of the Staphylococcus aureus isolates) [Table - 3]. Out of the 30 patients from whom MRSA was isolated, 18 had known risk factors for healthcare associated-MRSA. [14] The prevalence of the major known risk factors for the presence of healthcare associated-MRSA among the study population is presented in [Table - 4]. The other known risk factors, such as history of MRSA (nil), nonambulatory status (1 patient), requirement of chronic hemodialysis (nil), presence of a central venous catheter (nil), diabetes mellitus (nil), endotracheal intubation (1 patient), and household member with risk factors (6 patients), were seen in too small numbers to be assessed for significance.
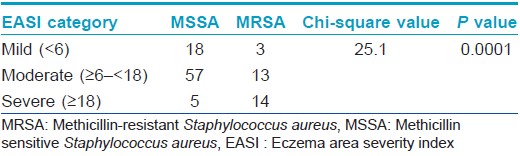
On comparing the disease severity in the groups in whom MRSA and methicillin sensitive Staphylococcus aureus were isolated, it was found that patients in the former group had significantly higher disease severity (P < 0.01), Mann-Whitney U test: [Table - 5] and [Table - 6]). Other factors like early age of onset, history of food allergies, seasonal exacerbation or inadequate breast feeding did not seem to influence disease severity. Comparison of patients in whom Staphylococcus aureus was isolated with those in whom it was not, did not reveal any significant difference in disease severity [Table - 5]. Despite detailed enquiries, no definite information regarding previous medications like topical steroids or calcineurin inhibitors was available in the majority due to a combination of poor record keeping and lack of awareness of the care-givers. Therefore, the association of these medications with disease severity could not be probed.
DISCUSSION
0Staphylococcus aureus is a gram-positive bacterium that produces a remarkable array of cell-surface and secreted virulence factors which facilitate disease causation. The organism develops drug resistance at pace with the development of new antimicrobial agents. [15] The organism often colonizes atopic dermatitis-damaged skin and the anterior nares. It spreads from these sites to infect other body sites. It is likely that most of the patients with atopic dermatitis are colonized with Staphylococcus aureus.This could be the result of various host factors such as skin-barrier dysfunction and deficiency of human antimicrobial peptides. Our study reports a high degree (92.4%) of Staphylococcus aureus colonization among atopic dermatitis patients, which is consistent with previous reports. [16],[17],[18] The rather high rates of isolation could be explained by the fact that these subjects were not treated with antibiotics recently. However, contrary to prior reports, no significant difference in disease severity was found between patients in whom Staphylococcus aureus was isolated and those whom it was not. This was possibly because the latter group was too small for a statistically significant difference to be noted.
Previous studies on atopic dermatitis differ widely in the proportion of cases colonized with MRSA depending on the geographic location where the study was conducted. In the study done at the Children′s Hospital, Philadelphia, MRSA was isolated from 13% of the study population (16% of Staphylococcus aureus isolates) [6] while Chung et al., in Korea reported a 13.9% prevalence of MRSA in atopic dermatitis (18.4% of Staphylococcus aureus isolates) [19] and Tang et al., in Taiwan, reported a 14.3% prevalence (30.8% of Staphylococcus aureus isolates). [20] The study conducted by Balma-Mena et al., in Toronto reported very low prevalence rates of 0.5%, [21] while that by Hill et al., in New Zealand reported a 2% prevalence. [22] Our finding of MRSA prevalence of 25.2% among the atopic dermatitis patients appears to be the highest so far and is a cause for concern. Further studies on Indian subjects are required to see if this alarming result is consistent over different centres and geographical areas of our country.
As our study was conducted in an outpatient setting, most of the strains isolated were expected to be community-acquired MRSA. Community acquired-MRSA strains are known to have existed since about two decades but have recently emerged as important pathogens worldwide. They carry the resistance genes in the staphylococcal chromosomal cassette mec IVA, which is smaller and encodes genes for methicillin resistance alone rather than multiple antibiotic resistance as seen in healthcare associated-MRSA. Nonetheless, recently, there are reports of multiple antibiotic-resistant community acquired-MRSA strains emerging from Asia. Some studies report that the differentiation of community acquired-MRSA from healthcare associated-MRSA has now become indistinct with the crossing over of community acquired strains into the healthcare settings. [8] In our study, of the 30 patients from whom MRSA was isolated, 18 had risk factors associated with healthcare associated-MRSA. This underscores the need for specific laboratory delineation for proper identification of the strains, irrespective of the disease setting.
It is recognized that hospitalized patients with MRSA suffer from worse outcomes when compared to patients infected with methicillin sensitive Staphylococcus aureus. Among community acquired-MRSA strains, Panton-Valentine leukocidin is identified as the major virulence factor. This factor is absent in methicillin sensitive Staphylococcus aureus. It has been suggested that the production of inflammatory cytolysins and high level superantigens by community acquired-MRSA increases their ability to cause extensive atopic dermatitis. [18] We found a strong association between the presence of MRSA and increased severity of atopic dermatitis (P < 0.01), whereas other factors like early age of onset, food allergies, seasonal exacerbation or inadequate breast feeding were not associated with disease severity. This result corroborates the theoretical possibility of MRSA causing more severe forms of disease. Conversely, it may also be reasoned that increased disease severity could be a marker for MRSA as there is a greater probability of these patients being hospitalized previously.
This study had certain limitations; differentiation of MRSA strains as community acquired or healthcare associated could not be done. Further, this being an observational study, the possibility of bias being introduced by unforeseen factors cannot be discounted.
In conclusion, our study reports a high prevalence of MRSA in pediatric atopic dermatitis and we demonstrate for the first time, significantly increased disease severity in atopic dermatitis patients from whom MRSA was isolated. These findings may have significant implications on the therapy of atopic dermatitis in future. With more than a quarter of atopic dermatitis patients harboring MRSA in our study, we need to establish precise guidelines for prescribing antibiotics, both oral and topical, to prevent further development of resistant strains. The possibility of MRSA causing increased disease severity needs to be explored in depth using study designs with a larger sample size. This may further elucidate the disease pathogenesis as well as open new windows of opportunities in treatment, especially in refractory or resistant cases.
| 1. |
Dhar S, Banerjee R. Atopic dermatitis in infants and children in India. Indian J Dermatol Venereol Leprol 2010;76:504-13.
[Google Scholar]
|
| 2. |
Leyden JJ, Marpes RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol 1974;90:525-30.
[Google Scholar]
|
| 3. |
Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: Colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 2002;147:55-61.
[Google Scholar]
|
| 4. |
D'Souza N, Rodrigues C, Mehta A. Molecular characterization of Methicillin-resistant Staphylococcus aureus with emergence of epidemic clones of sequence type (ST) 22 and ST 772 in Mumbai, India. J Clin Microbiol 2010;48:1806-11.
Staphylococcus aureus with emergence of epidemic clones of sequence type (ST) 22 and ST 772 in Mumbai, India. J Clin Microbiol 2010;48:1806-11.'>[Google Scholar]
|
| 5. |
Buescher ES. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr Opin Pediatr 2005;17:67-70.
[Google Scholar]
|
| 6. |
Suh L, Coffin S, Leckerman KH, Gelfand JM, Honig PJ, Yan AC. Methicillin-resistant Staphylococcus aureus colonisation in children with atopic dermatitis. Pediatr Dermatol 2008;25:528-34.
[Google Scholar]
|
| 7. |
Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence and susceptibility pattern. Indian J Med Res 2013;137:363-9.
[Google Scholar]
|
| 8. |
Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol 2007;56:1-16.
[Google Scholar]
|
| 9. |
Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatovener 1980;S92:44-7.
[Google Scholar]
|
| 10. |
Tofte S, Graeber M, Cherill M, Omoto M, Thurston M, Hanifin J. Eczema area and severity index (EASI): A new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol 1998;11:S197.
[Google Scholar]
|
| 11. |
Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10:11-8.
[Google Scholar]
|
| 12. |
Barbier N, Paul C, Luger T, Allen R, De Prost Y, Papp K, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol 2004;150:96-102.
[Google Scholar]
|
| 13. |
Charman C, Williams H. Outcome measures of disease severity in atopic dermatitis. Arch Dermatol 2000;136:763-9.
[Google Scholar]
|
| 14. |
Tacconelli E, Venkataraman L, De Girolami PC, D'Agata EMC. Methicillin-resistant Staphylococcus aureus bacteraemia diagnosed at hospital admission: Distinguishing between community-acquired versus healthcare-associated strains. J Antimicrob Chemother 2004;53:474-9.
Staphylococcus aureus bacteraemia diagnosed at hospital admission: Distinguishing between community-acquired versus healthcare-associated strains. J Antimicrob Chemother 2004;53:474-9.'>[Google Scholar]
|
| 15. |
Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998;339:520-32.
[Google Scholar]
|
| 16. |
Monti G, Tonetto P, Mostert M, Oggero R. Staphylococcus aureus skin colonization in infants with atopic dermatitis. Dermatology 1996;193:83-7.
[Google Scholar]
|
| 17. |
Leung DY. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J Allergy Clin Immunol 2000;105:860-76.
[Google Scholar]
|
| 18. |
Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus and its relevance to Atopic dermatitis. J Allergy Clin Immunol 2010;125:39-49.
[Google Scholar]
|
| 19. |
Chung HJ, Jeon HS, Sung H, Kim MN, Hong SJ. Epidemiological characteristics of methicillin-resistant Staphylococcus aureus isolates from children with eczematous atopic dermatitis lesions. J Clin Microbiol 2008;46:991-5.
[Google Scholar]
|
| 20. |
Tang CS, Wang CC, Huang CF, Chen SJ, Tseng MH, Lo WT. Antimicrobial susceptibility of Staphylococcus aureus in children with atopic dermatitis. Pediatr Int 2011;53:363-7.
[Google Scholar]
|
| 21. |
Balma-Mena A, Lara-Corrales I, Zeller J, Richardson S, McGavin MJ, Weinstein M, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: A cross-sectional study. Int J Dermatol 2011;50:682-8.
[Google Scholar]
|
| 22. |
Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: A New Zealand experience. Australas J Dermatol 2011;52:27-31.
[Google Scholar]
|
Fulltext Views
4,827
PDF downloads
2,119





