Translate this page into:
Methotrexate monotherapy versus methotrexate and apremilast combination therapy in the treatment of palmoplantar psoriasis: A prospective, randomised, assessor-blinded, comparative study
-
Received: ,
Accepted: ,
How to cite this article: Hassanandani T, Panda M, Jena AK, Raj C. Methotrexate monotherapy versus methotrexate and apremilast combination therapy in the treatment of palmoplantar psoriasis: A prospective, randomised, assessor-blinded, comparative study. Indian J Dermatol Venereol Leprol 2023;89:213-20.
Abstract
Background:
Palmoplantar psoriasis is a chronic debilitating condition which significantly impairs quality of life.
Objectives:
To assess the efficacy and safety of the combination of apremilast and methotrexate compared with methotrexate monotherapy in the treatment of palmoplantar psoriasis. Also, to study the impact on treatment on the Dermatology Life Quality Index and Palmoplantar Quality of Life Index.
Methods:
A total of 64 patients were randomised to two groups in a 1:1 ratio - Group A received both methotrexate and apremilast in combination, while Group B received only methotrexate, for 16 weeks. The primary endpoints were the mean score of Modified Palmoplantar Psoriasis Area and Severity Index at week 16, the proportion of patients achieving modified palmoplantar psoriasis area severity index-75 and/or Palmoplantar Psoriasis Physician Global Assessment score 0/1 at week 16.
Results:
A significantly higher proportion of patients in Group A achieved Modified Palmoplantar Psoriasis Area and Severity Index-75 at week 16 (43% in Group A vs 30% in Group B). The Modified Palmoplantar Psoriasis Area and Severity Index score was significantly lower in the combination group at week 16 (4.03 ± 2.05 in Group A and 5.89 ± 2.31 in Group B, P-value = 0.002). About 80% of patients in the combination group with baseline Palmoplantar Psoriasis Physician Global Assessment ≥3 achieved Palmoplantar Psoriasis Physician Global Assessment 0/1 compared to 60% in Group B. The combination group showed a significantly higher reduction in Dermatology Life Quality Index and Palmoplantar Quality of Life Index scores compared to the methotrexate alone group (P-value = 0.025). No notable adverse events were observed.
Limitation:
The limitations of the study were single blinding, small sample size and a lack of longer follow up to assess the rate of relapse. We did not account for attrition during sample size calculation. Also, due to the paucity of data regarding the use of apremilast in palmoplantar psoriasis, definitive comparisons could not be made with previous studies.
Conclusion:
The combination of apremilast and methotrexate has superior efficacy and a similar safety profile as compared to methotrexate monotherapy for the treatment of moderate to severe palmoplantar psoriasis.
Keywords
Methotrexate
apremilast
treatment of psoriasis
palmoplantar psoriasis
Plain Language Summary
Psoriasis is a chronic disorder of the skin which often leads to mental distress in patients due to its recurring/relapsing nature multiplied with social stigma due to ignorance. Palmoplantar psoriasis is a type of localized psoriasis which is known to be resistant to treatment. Prompt diagnosis and aggressive treatment provides relief to the patient and may help in avoiding further complications. Though monotherapy is the first choice of clinicians, combination therapy is always explored in the hope to achieve better results. As methotrexate and apremilast have different mechanisms of action and there is a paucity of literature on combination therapy of these two molecules in the treatment of psoriasis, we co-administered these two molecules and compared with methotrexate monotherapy. We found that the combination therapy had superior efficacy and a similar safety profile as compared to only methotrexate for the treatment of moderate to severe palmoplantar psoriasis.
Introduction
Psoriasis is a multifactorial, chronic, papulosquamous disorder affecting nearly 2–3 % of the population worldwide and upto 8% of the population in certain countries. Nearly 13–15% of all psoriasis cases present as palmoplantar psoriasis, clinically characterised by well-defined erythema, scaling and fissuring involving the palms and soles. Although palmoplantar involvement contributes to only a small proportion of body surface area, it can severely affect the patient’s quality of life and critically impair productivity at work. The treatment options include super-potent topical steroids alone or combined with calcipotriene in mild cases and systemic therapy in moderate to severe cases. Adisen et al.1 in a retrospective analysis showed that only 27.4% of patients improved with topical agents while others needed additional systemic therapies. Despite the obvious need for systemic therapies, very few clinical trials have been done in this regard. The various agents that have to date shown promising results include alefacept,2 secukinumab,3 infliximab,4 acitretin and methotrexate.5 The cost of these therapies further adds to the burden of an already debilitating condition.6 In a developing country like India, biologics are beyond the reach of the general population due to financial constraints and most insurance companies refuse to bear the expenses of biologic treatment. This propels the constant search for affordable and effective therapy for palmoplantar psoriasis.
Apremilast is a new oral small molecule phosphodiesterase 4 inhibitor, which has been approved for the treatment of psoriasis and psoriatic arthritis in adults. The efficacy has been established in two phase III clinical trials (ESTEEM and PALACE).7,8
Methotrexate is one of the oldest known systemic agents used in psoriasis. However, palmoplantar psoriasis is inherently resistant to treatment and has shown less than gratifying results with methotrexate. In a study from India by Janagond et al.9 only 24% of patients achieved Modified Palmoplantar Psoriasis Area and Severity Index-75 (m-PPPASI-75) at the end of 12 weeks of oral methotrexate. On the other hand, combination therapies with methotrexate and topical PUVAsol,10 narrow-band UVB therapy,11 and topical halobetasol ointment in palmoplantar psoriasis have shown better results when compared to monotherapy.
Methotrexate and apremilast have different mechanisms of action and adverse effect profiles; this led us to hypothesize that combining them might provide a synergistic effect, with probable superior efficacy compared to monotherapy with these agents in palmoplantar psoriasis.
In a study by Liu et al.12 it was observed that the Cmax (peak concentration) and AUC (area under the drug concentration-time curve) parameters for apremilast and methotrexate, individually and after co-administration, were all within the FDA acceptance range, proving that apremilast and methotrexate can be combined without any effect on the pharmacokinetics of the either drug.
Various quality of life measures has been devised to assess the detrimental impact of psoriasis on life. The Palmoplantar Quality of Life Index (ppQLI) described by Farley et al.13 is one such system.
We undertook this study due to the scarcity of randomised controlled trials evaluating the effectiveness of the combination of methotrexate and apremilast in the treatment of palmoplantar psoriasis and its effect on the Dermatology Life Quality Index (DLQI) as well as the ppQLI.
Materials and Methods
A comparative, randomised, assessor-blinded, prospective study was undertaken on patients with palmoplantar psoriasis seen at the department of dermatology at the Institute of Medical Sciences & SUM Hospital, Bhubaneswar. Institutional ethical committee clearance was obtained prior to trial commencement. The trial was registered in the Clinical Trial Registry of India with Registration number: CTRI/026379.
The study period was from July 2020 to May 2021.
All study participants were informed about the study objective and informed consent was taken in their preferred (English/local) language. Minors were not included in this study. The option to opt out of the study was kept open without any clause. Data collected was kept confidential.
Inclusion criteria: Histopathologically (characteristic features of psoriasis, i.e., hyperkeratosis, parakeratosis, Munro’s microabscess, Kogoj’s spongiform pustules, elongation of rete ridges, suprapapillary thinning, hypogranulosis and dilated vessels in the tips of the papillae) and dermoscopically (white scales, regularly arranged dots and globular vessels) proven palmoplantar psoriasis with:
Modified Palmoplantar Psoriasis Area and Severity Index (m-PPPASI) ≥10,
Palmoplantar Psoriasis Physician Global Assessment (PPPPGA) ≥3 on a 5-point scale
DLQI ≥10.
 |
m-PPPASI: Modified palmoplantar psoriasis area and severity index, PPPPGA: Palmoplantar psoriasis physician global assessment, DLQI: Dermatology life quality index, ppQLI: Palmoplantar quality of life index
Exclusion criteria: Patients with deranged complete blood count (CBC), liver function test (LFT) or renal function, pregnancy and lactation, alcoholics, palmoplantar pustulosis, erythrodermic psoriasis, guttate psoriasis, generalised pustular psoriasis, chronic or recurrent infections, including tuberculosis and history of a lymphoproliferative disorder.
Sample size
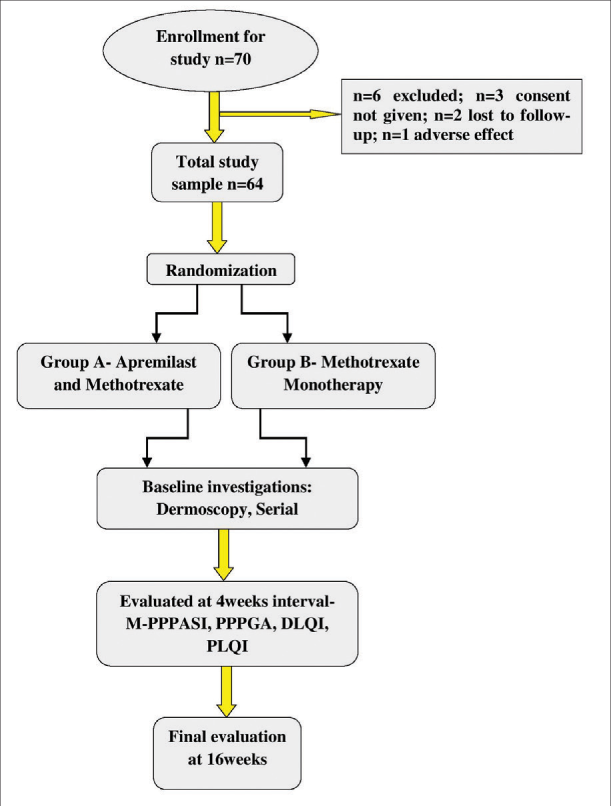
Using the Raosoft sample size calculator, the estimated population size was 80 with 95% confidence interval for the total study period of 18 months, the estimated sample size obtained was 67. Seventy patients were considered for enrolment in the study, out of which, six were excluded: three did not give consent to enrol, two were lost to follow up after the first visit and one withdrew from the study due to adverse effects in the first week. Sixty four were then randomly allocated into two groups of 32 patients each. Group A received a combination of apremilast with methotrexate while Group B received methotrexate alone.
Each patient was subjected to thorough clinical general systemic and dermatological examination. There was a washout period of 14 days for topical treatment and 28 days for phototherapy or systemic drugs. None of the patients had been on any biologic treatment previously. Dermoscopic evaluation of the lesions was done at baseline and at the end of Week 16 using a handheld DermLite 3 dermoscope and pictures were taken using iPhone X. Skin biopsies were done at baseline to confirm the diagnosis. CBC, LFT, serum urea and creatinine, chest X-rays were done at the baseline and repeated at monthly intervals. After March 2020, all the patients were subjected to COVID-19 testing before the commencement of therapy in light of the ongoing pandemic at the time. Female patients in the reproductive age-group underwent a compulsory pregnancy test and were advised to use physical contraceptive measures while on medication and for 1 menstrual cycle after stopping methotrexate. Males were advised contraception till three months after discontinuation of methotrexate. The baseline demographic data is presented in Table 2.
| GROUP A METHOTREXATE + APREMILAST |
GROUP B METHOTREXATE |
P-value | |
|---|---|---|---|
| Mean Age (years) Age ≥40 N(%) Age <40 N(%) |
40.87 ± 10.57 16 (53.3) 14 (46.7) |
44.47 ± 9.61 21 (70) 9 (30) |
0.173 |
| Gender Male Female |
17 (54.8) 13 (43.3) |
16 (55.2) 14 (45.2) |
0.606 |
| Duration of illness (in years; Mean ± SD) >5 years n(%) <5 years n(%) |
6.33 ± 3.05 18 (60) 12 (40) |
7.86 ± 2.96 22 (73.3) 8 (26.7) |
0.355 |
| Mean age at onset | 34.53 ± 10.005 | 36.60 ± 11.22 | 0.455 |
| Baseline weight (in kg) | 75.73 ± 9.23 | 71.63 ± 11.23 | 0.128 |
| BMI | 25.02 ± 3.91 | 23.65 ± 4.74 | 0.227 |
| Smoking n(%) | 6 (54.5) | 5 (45.5) | 1.000 |
| Diabetes n(%) | 10 (33.3) | 10 (33.3) | 1.000 |
| Hypertension n (%) | 9 (30) | 7 (23.3) | 0.158 |
| Hyperlipidaemia n(%) | 1 (3.3) | 2 (6.7) | 1.000 |
| Obesity n(%) | 2 (6.7) | 4 (13.3) | 0.612 |
| Hypothyroidism n(%) | 3 (10) | 5 (16.7) | 0.424 |
| Family history n(%) | 4 (13.3) | 4 (13.3) | 1.000 |
| Joint involvement n(%) | 3 (10) | 3 (10) | 1.000 |
| Nail involvement n(%) | 15 (50) | 16 (53.3) | 1.000 |
| Previous treatment taken n (%) | 16 (53.3) | 14 (46.7) | 0.600 |
BMI: Body mass index
Randomisation:
The patients were randomised 1:1 on Day 0 to receive either a combination of apremilast and methotrexate or methotrexate alone. Randomisation was done using a computer-generated randomisation sequence. The participants were enrolled and assigned to the intervention by a dermatologist who was not involved in the assessment.
The dose of Apremilast used was 30 mg twice daily. Initial dose titration was done. The first dose of 10 mg was given on Day 0 and the dose was gradually increased. On Day 5 patients received 30 mg twice daily, to be continued for a duration of 16 weeks. Methotrexate was given as weekly doses of 0.2 mg/kg. Folic acid (1 mg) supplementation was given daily except on the days when methotrexate was given.
The patients were advised to use only bland emollients during the study period. Oral anti-histamines were advised when required.
The patients were advised to follow-up every four weeks for 16 weeks, i.e., a total of five visits. High-resolution photographs of the patients were taken at the same distance and with the same position using a digital camera at baseline and then at every subsequent visit.
All the patients were analysed by two independent dermatologists who were blinded to the treatment protocol and trained to calculate PPPPGA and m-PPPASI.
Evaluation of treatment:
Efficacy of the treatments was evaluated using the m-PPPASI and PPPPGA.
Quality of life was evaluated with the help of DLQI and ppQLI
Primary endpoints:
The mean m-PPPASI scores of Group A and Group B at week 16.
Proportion of patients in Group A and B achieving at least a 75% reduction in baseline m-PPPASI , i.e., m-PPPASI-75 at week 16.
Patients who achieved PPPPGA scores of 0/1 at Week 16 in Group A and Group B.
Secondary endpoints:
The reduction in mean m-PPPASI in Group A and B at weeks 4, 8, 12 and 16.
Exploratory endpoints:
Change from baseline in DLQI and ppQLI scores at Week 16.
Safety analysis was done by recording and evaluating the adverse events.
Statistical analysis
Baseline demographics, improvement in m-PPPASI, DLQI and ppQLI at week 16, and safety data are presented as mean and standard deviation (SD) with 95% confidence intervals. They were analysed using the paired t-test. The proportions of patients who achieved a m-PPPASI-75 and PPPPGA of 0/1 at Week 16 are presented as percentages with 95% confidence intervals and the analysis was done using the Chi-square test. Comparisons between the two groups in terms of m-PPPASI, DLQI and ppQLI were performed using the independent t-test. All statistical tests were two-sided and performed at a significance level of 0.05. Analyses were performed using IBM SPSS software version 26.0.0 (Statistical Package for Social Sciences, IBM SPSS Inc., Chicago, IL) and Microsoft Excel.
The flow of patients is given in Table 1.
Results
The mean age was 40.87 ± 10.57 and 44.47 ± 9.61 years in Groups A and B, respectively. The mean duration of psoriasis was 6.33 ± 3.05 years and 7.86 ± 2.96 years in Groups A and B, respectively. Out of the 30 patients analysed in Group A, 17 (54.8%) were males and 13 (43.3%) were females, likewise in Group B, out of the 30 patients, 16 (55.2%) were males and 14 (45.2%) were females. The mean weight (in kg) at baseline was 75.73 ± 9.23 and 71.63 ± 11.23 in Groups A and B, respectively. The two groups were comparable at baseline [Table 2].
Mean m-PPPASI score at week 16
A statistically significant reduction was observed in the m-PPPASI at Week 16 in Group A (P = 0.001).
There was also a statistically significant reduction in the m-PPPASI from baseline to Week 16 in Group B (P = 0.001).
However, the mean m-PPPASI values at week 16 were significantly higher in Group B (methotrexate alone), when compared to Group A (methotrexate + apremilast). The difference in means between Groups A and B was statistically significant (P = 0.002) at week 16 [Table 3 and Figure 1].
| Mean m-PPPASI score | Group A (Methotrexate + Apremilast) |
Group B (Methotrexate) | P-value |
|---|---|---|---|
| Baseline | 21.73 ± 2.70 | 20.83 ± 2.41 | 0.181 |
| Week 4 | 18.23 ± 2.09 | 19.74 ± 2.10 | 0.007 |
| Week 8 | 11.48 ± 2.28 | 16.68 ± 2.43 | 0.000 |
| Week 12 | 6.06 ± 2.70 | 12.53 ± 2.73 | 0.000 |
| Week 16 | 4.03 ± 2.05 | 5.89 ± 2.31 | 0.002 |
| P-value | 0.001 | 0.001 |
m-PPPASI: Modified palmoplantar psoriasis area and severity index
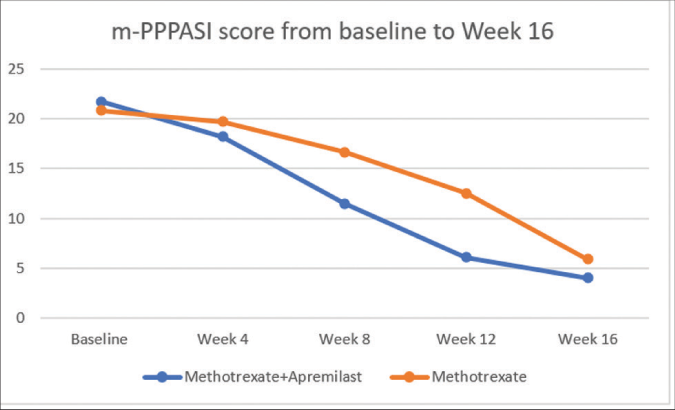
- Modified Palmoplantar Psoriasis Area and Severity Index score from baseline to Week 16
m-PPPASI-75 at week 16
The fraction of patients who achieved m-PPPASI-75 at week 16 were 13 (43%) in Group A, and 9 (30%) in Group B; the difference was statistically significant (P = 0.001) [Figures 2 and 3].

- Pre-treatment photo of patient in Group A

- Pre-treatment photo of patient in Group A

- Post-treatment photo of patient in Group B

- Pre-treatment photo of patient in Group B
PPPPGA score 0/1 at week 16
The number of patients with PPPPGA ≥3 who achieved PPPPGA 0/1 at week 16 was higher at 24 (80%) in Group A and 18 (60%) in Group B. This difference was statistically significant. [Figure 4].
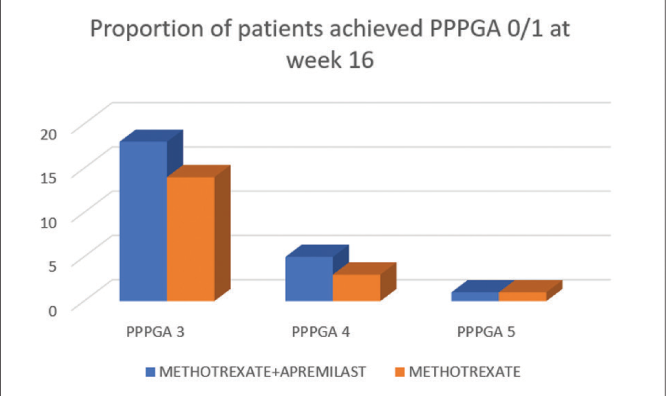
- Proportion of patients who achieved PPPPGA 0/1 at week 16 in Group A and B
DLQI score
In Group A, the mean DLQI at baseline was 15.9 ± 2.70 and at the end of 16 weeks it was 5.26 ± 1.43. There was a significant decrease in the DLQI post-treatment (P = 0.000).
In Group B, the mean DLQI at baseline was 16.53 ± 2.55 and at the end of 16 weeks it was 6.8 ± 2.15. There was a significant decrease in the DLQI after treatment (P = 0.000).
The mean DLQI at week 16 was 5.26 ± 1.43 in Group A and 6.80 ± 2.15 in Group B and the difference was highly significant (P = 0.001).
ppQLI score
The mean reduction in ppQLI score of hands from baseline to week 16 was 31.43 ± 7.36 in Group A and 27.13 ± 7.07 in Group B. The mean reduction of ppQLI was higher in Group A and the difference between the two groups was statistically significant (P = 0.025).
The mean reduction in ppQLI score of feet from baseline to week 16 was 29.23 ± 7.48 in Group A and 24.13 ± 7.09 in Group B. Mean reduction of ppQLI was higher in Group A and the difference between the two groups was statistically significant (P = 0.009).
Thus the reduction of ppQLI scores for both hands and feet was higher in Group A as compared to Group B. Also, the score reduction was significantly higher for hands as compared to feet in both groups.
However, the difference between reduction in palmoplantar quality of life index score for hand and feet was higher in Group B as compared to Group A [Table 4].
| Group A BASELINE |
WEEK 16 | Group B BASELINE |
WEEK 16 | |
|---|---|---|---|---|
| HANDS | 43.16 ± 7.40 | 11.73 ± 1.43 | 42.60 ± 6.28 | 15.46 ± 2.51 |
| FEET | 41.21 ± 7.5 | 11.93 ± 1.59 | 40.82 ± 6.41 | 16.46 ± 2.66 |
ppQLI: Palmoplantar quality of life index
Safety analysis
The most common adverse effect seen was diarrhoea in 8 (26.6%) patients in Group A and in 5 (16.6%) patients in Group B.
In Group A it was commonly reported in the first two weeks after starting therapy and in most patients, it was mild to moderate with resolution within 3–4 weeks. Two patients needed oral fluids and loperamide for the management of diarrhoea.
The other common side effects were nausea and vomiting, headache and gastrointestinal intolerance. Other rare side effects noted were upper respiratory tract infection, mood disorder, and abnormal liver function. All the adverse events were managed conservatively [Table 5].
| Adverse effects | Group A (Methotrexate+Apremilast) | Group B (Methotrexate) |
|---|---|---|
| Nausea & vomiting | 7 | 5 |
| Diarrhoea | 8 | 5 |
| Headache | 4 | 2 |
| Gastrointestinal intolerance | 2 | 2 |
| Upper respiratory tract infections | 1 | 0 |
| Abnormal liver function test | 1 | 2 |
| Mood disorder | 1 | 0 |
The mean weight (in kg) at baseline in Group A was 75.73 ± 9.23 and at the end of 16 weeks it was 74.10 ± 8.42. The reduction of weight in Group A was statistically significant (P = 0.001). The mean weight (in kg) at baseline in Group B was 71.63 ± 11.22 and at the end of 16 weeks it was 71.60 ± 11.51; the reduction of weight in Group B was not statistically significant (P = 0.912).
Discussion
Palmoplantar psoriasis is a chronic crippling condition with a considerable effect on the patient’s quality of life and work productivity. Conventional therapies approved for the treatment of psoriasis seldom show efficacy in this difficult to treat area. Hence, newer treatment modalities are a need of the hour. Combination therapies in psoriasis are expected to be beneficial as they improve the efficacy with faster clearance and reduce the side effects that are seen with prolonged treatment. Oral methotrexate and apremilast is one such combination, where both the drugs have different mechanisms of action, possibly leading to a better outcome. Apremilast, having the advantage of rare major side effects and negligible monitoring requirements, is an ideal molecule to be combined with methotrexate, which has been the mainstay of therapy in psoriasis for decades.
In a study by Bisonnette et al.14 only 22% patients randomised to apremilast achieved Modified ppPAsI-75 at week 16, while in a study by Janagond et al.10 24% patients in methotrexate group had achieved Modified ppPASI-75 at week 12. In our study, a significantly higher proportion of patients achieved Modified ppPASI-75 at week 16 in the combination group (43% Group A vs 30% in Group B).
The reduction in m-PPPASI from baseline was maximum between 1st and 2nd follow-up visits i.e., between week 4 and 8 (6.09 ± 2.10) in Group A. Whereas in Group B, the maximum reduction was seen between the 3rd and 4th visits, i.e., week 12 and 16 (7.53 ± 1.62). Combination therapy helped in achieving a faster reduction in m-PPPASI. This was similar to the report by Ständer et al. where patients with palmoplantar pustular psoriasis achieved excellent improvement in Physician Global Assessment as early as four weeks after starting apremilast.15
There was also a significant reduction in the PPPPGA score in both the groups at week 16, however, the reduction was statistically more significant in Group A where 80% of patients with baseline PPPPGA ≥3 achieved Palmoplantar Psoriasis Physician Global Assessment 0/1 compared to 60% in Group B. In a study by Bissonnette et al.4 48% of the patients who took apremilast with baseline PPPPGA score ≥3 achieved PPPPGA score 0/1 in comparison to 27% in the placebo group at week 16 (P = 0.021).
Similarly, the DLQI scores improved significantly in both the groups, but the improvement was more in the combination group compared to methotrexate alone. Similar findings were seen for ppQLI. The mean reduction of ppQLI scores for hands was higher in Group A and the difference was statistically significant (P = 0.025). The mean reduction of ppQLI scores for feet was higher in Group A and the difference between two groups was statistically significant (P = 0.009). On comparison, the reduction of ppQLI score of hands was more than that of feet in both groups.
A secondary aim of our study was to compare the safety profiles among patients treated with a combination of methotrexate and apremilast or methotrexate alone.
The majority of our patients experienced minor side effects which were tolerable and were managed with conservative treatment. Interestingly, the frequency of nausea and/or diarrhoea in Group A, was not higher than the monotherapy group. In a study by AbuHilal et al.,16 similar findings were observed where 20% of patients on methotrexate/apremilast combination sub-group complained of nausea and/or diarrhoea compared to 30% in all groups combined.
In our study, patients in Group A experienced weight loss. In previous studies with apremilast, a 5–10 % decrease in body weight was observed in nearly twice as many patients taking apremilast than placebo during trials in patients with psoriasis (12 vs. 5%). The mean weight loss at the end of 52 weeks of apremilast was 1.99 kg in the pooled data from ESTEEM 1 and 2 trials.6 However, nearly 50% of patients with weight loss, did not report other gastrointestinal adverse effects, which is similar to findings by Abu Hilal et al.16 Hence, it can be inferred that the weight loss is not completely related to gastrointestinal adverse effects and is mediated through other mechanisms. Studies using murine models demonstrated an association between PDE4 inhibition and adiposity.17 Also, PDE4 inhibition has been linked to increased cellular cholesterol efflux by inducing ABCA1 transporter18 possibly explaining the weight loss in patients taking apremilast.
Methotrexate and apremilast have different mechanism of action and adverse effect profiles. Thus, they can be combined to provide a synergistic effect, with probable superior efficacy compared to monotherapy with these agents in patients with palmoplantar psoriasis.
In our study, there were no severe interactions or adverse events seen due to co-administration of both the drugs. Hence, it can be inferred that the combination was safe and effective in the treatment of palmoplantar psoriasis.
The limitations of the study were single blinding (only assessor) and a small sample size. A longer follow-up was required to assess the rate of relapse. We did not account for attrition during sample size calculation. Also, due to the paucity of data regarding the use of Apremilast in palmoplantar psoriasis, definitive comparisons could not be made with previous studies.
Declaration of patient consent
Patient’s consent is not required as the patient’s identity is not disclosed or compromised.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- A retrospective analysis of treatment responses of palmoplantar psoriasis in 114 patients. J Eur Acad Dermatol Venereol. 2009;23:814-9.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic hotline. Alefacept in the treatment of hyperkeratotic palmoplantar psoriasis. Dermatol Ther. 2010;23:556-60.
- [CrossRef] [PubMed] [Google Scholar]
- Secukinumab shows significant efficacy in palmoplantar Methotrexate and apremilast in palmoplantar psoriasis psoriasis: Results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of palmoplantar psoriasis with infliximab: A randomized, double-blind placebo-controlled study. J Eur Acad Dermatol. 2011;25:1402-8.
- [CrossRef] [PubMed] [Google Scholar]
- Low dose acitretin versus methotrexate in the treatment of palmoplantar psoriasis: A case series. J Eur Acad Dermatol Venereol. 2019;33:e246-7.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: Results of a pooled analysis from phase II PSOR-005 and phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2016;75:99-105.
- [CrossRef] [PubMed] [Google Scholar]
- Incremental cost per responder (PASI-75, PASI-90 and PASI-100) based on a network meta-analysis of biologic therapies for psoriasis: Spain 2018. Actas Dermosifiliogr. 2019;110:517-8.
- [CrossRef] [PubMed] [Google Scholar]
- A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: Results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-34.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: A prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:e384-9.
- [CrossRef] [PubMed] [Google Scholar]
- Combination topical PUVAsol with methotrexate versus methotrexate in the treatment of palmoplantar psoriasis. Kathmandu Univ Med J. 2016;14:362-6.
- [Google Scholar]
- Comparative therapeutic evaluation of different topicals and narrow band ultraviolet B therapy combined with systemic methotrexate in the treatment of palmoplantar psoriasis. Indian J Dermatol. 2011;56:165-70.
- [CrossRef] [PubMed] [Google Scholar]
- The pharmacokinetic effect of coadministration of apremilast and methotrexate in individuals with rheumatoid arthritis and psoriatic arthritis. Clin Pharmacol Drug Dev. 2014;3:456-65.
- [CrossRef] [PubMed] [Google Scholar]
- Palmoplantar psoriasis: A phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-31.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: Results from a double-blind, placebo-controlled, randomized study. J Eur Acad Dermatol Venereol. 2018;32:403-10.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of refractory palmoplantar pustular psoriasis with apremilast: A case series. Front Med. 2020;7:543944.
- [CrossRef] [PubMed] [Google Scholar]
- Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis. J Cutan Med Surg. 2016;20:313-6.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology. 2009;150:3076-82.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclic AMP-specific phosphodiesterase 4 inhibitors promote ABCA1 expression and cholesterol efflux. Biochem Biophys Res Commun. 2002;290:663-9.
- [CrossRef] [PubMed] [Google Scholar]






