Translate this page into:
MIP vaccine in leprosy: A scoping review and future horizons
Corresponding author: Dr. Sunil Dogra, Department of Dermatology, Venereology and Leprology, PGIMER, Chandigarh, India. sundogra@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Narang T, Jain S, Kaushal I, Dogra S. MIP vaccine in leprosy: A scoping review and future horizons. Indian J Dermatol Venereol Leprol. 2024;90:606-14. doi: 10.25259/IJDVL_1172_2023
Abstract
Mycobacterium Indicus Pranii (MIP) vaccine is a killed vaccine developed in India for leprosy with immunotherapeutic as well as immunoprophylactic effects. MIP, earlier known as Mycobacterium welchii, is a rapidly growing non-pathogenic mycobacterium. The novelty of this bacterium is due to its translational application as an immunotherapeutic agent. When administered intradermally, the vaccine induces cell-mediated immunity in the host towards Mycobacterium leprae. It leads to faster clinical and histopathological improvement, rapid bacillary clearance, and also lepromin conversion in anergic leprosy patients. The beneficial role of the MIP vaccine in augmenting the therapeutic efficacy of Multidrug Therapy (MDT), particularly in highly bacillated leprosy patients, is well documented in various studies from India. The role of the vaccine in reactional states is controversial, with varied results in different studies. Overall, it is found to decrease the frequency of type 2 lepra reactions and is useful in recalcitrant erythema nodosum leprosum. Even though there may be an increased likelihood of type 1 reactions, no additional nerve function impairment is attributed to the vaccine in various studies. In household contacts of leprosy who are administered MIP, it is noted to confer protection from disease lasting up to 10 years. It may prove to be a cost-effective strategy in national leprosy programmes. Apart from local injection site reactions, the vaccine is relatively safe, but it is not recommended in pregnancy and lactation. This article provides an overview of the MIP vaccine’s clinical application in the context of leprosy spanning over 40 years. It also considers the vaccine’s possible future applications in the management of disease-related complications and achieving the long-term goal of zero leprosy.
Keywords
MIP
vaccine
leprosy
reactions
immunoprophylaxis
Introduction
Vaccines are an asset in the control of leprosy, because of their immunoprophylactic and immunotherapeutic properties. Unlike conventional chemotherapy, vaccines have the advantage of inducing immunologic memory, providing prolonged protection against the disease. The Global Leprosy Strategy 2021–2030, aiming for ‘Towards Zero Leprosy’, underscores the crucial need for preventing leprosy and associated deformities.1 Effective vaccination strategies play a pivotal role in achieving this objective. Although the World health organisation’s (WHO) introduction of Multidrug Therapy (MDT) in 1982 has proven highly efficacious in treating leprosy thereby significantly reducing its incidence and prevalence, India reported 1,03,819 cases in 2022, reflecting a 37% increase from the previous year. Besides checking transmission, vaccines may also help in decreasing the morbidity due to reactions that are triggered by both live and dead bacilli, as vaccines enhance the immunological clearance of dead Mycobacterium leprae (M. leprae). Despite numerous attempts with various vaccines over the years, the inability to cultivate M. leprae in artificial media has been the biggest roadblock in pursuit of an ideal leprosy vaccine. Vaccines for leprosy belong to three categories: the first category includes M. leprae-based vaccines like killed M. leprae, M. leprae + Bacillus Calmette–Guérin (BCG) and acetoacetylated M. leprae; the second category includes BCG, BCG + M. vaccae, Indian Cancer Research Centre (ICRC) bacillus, Mycobacterium habana and Mycobacterium Indicus Pranii (MIP), which are cultivable mycobacteria, and the third is LepVax, a subunit vaccine.2
Mycobacterium Indicus Pranii, formerly known as Mycobacterium w (M.w), is a non-pathogenic and rapidly growing atypical mycobacterium that can be easily cultivated in the laboratory. It was developed (1980s) in India for use in leprosy by Dr. Pran Talwar at the National Institute of Immunology.3 MIP has both immunotherapeutic and immunoprophylactic potential in the management of leprosy. This article is a scoping review of the MIP vaccine’s clinical utility within the context of leprosy, spanning over the past four decades, while contemplating its potential future in leprosy eradication and management of leprosy complications such as recalcitrant/chronic erythema nodosum leprosum (ENL) and prevention of deformities.
A recently published article provides an in-depth account of the history, pharmacology, mechanism of action and clinical application of MIP in various dermatological indications.4
Mechanism of Action in Leprosy
In leprosy, an aberrant immune response plays a key role in the development of the disease’s symptoms and complications. Patients with lepromatous leprosy (LL) are anergic to M. leprae and are at increased risk of reinfection even after completion of treatment.5
MIP vaccine elicits innate and T cell immune responses in anergic LL patients [Figure 1]. These T cell responses develop against low molecular weight 14–45 kDa entities of the MIP vaccine.6,7 Apart from LL Hansen, T cells from tuberculoid patients and healthy contacts also mount a response against many antigens of the MIP vaccine. The immunodominant 28–31 kDa antigenic fraction of the bacteria carries T cell triggering determinants which activate Th1/Th17 pathways.8 Thus, it is useful as immunotherapy in all spectrums of leprosy and as an immunoprophylactic agent in healthy contacts.
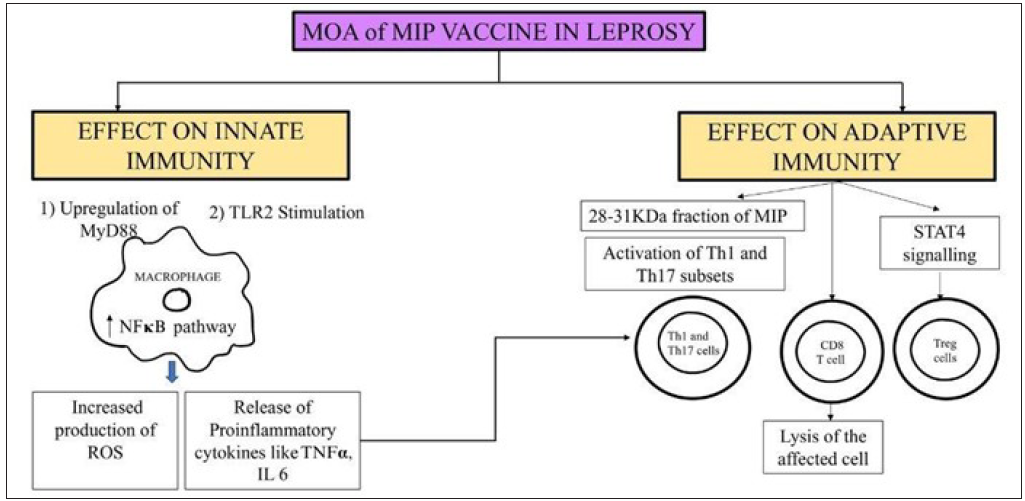
- Schematic diagram of the mechanism of action of MIP vaccine in leprosy. (ROS: Reactive oxygen species, TNF α: Tumor necrosis factor-alpha, TLR: Toll-like receptor, MIP: Mycobacterium Indicus Pranii, Th: T helper cells, STAT: Signal transducers and activators of transcription, MOA: Mechanism of action, IL: Interleukin, TLR: Toll-like receptor, MyD: Myeloid Differentiation primary response 88, NF: Nuclear factor kappa beta.)
Role in immunotherapy
The advent of MDT in 1982 was a milestone in the treatment of leprosy. While MDT initially resulted in a significant reduction in the incidence and prevalence of leprosy, the new case detection rate eventually reached a plateau. This could be linked to undiagnosed high bacilliferous patients, especially the ones belonging to LL or borderline lepromatous (BL) spectrum, who have the potential to transmit the disease. Some of these individuals may continue to harbour viable bacilli even after completing the fixed duration of treatment, rendering them susceptible to relapses and transmitting the disease to contacts.2 Patients with a high bacillary load need immunotherapy to circumvent the immunological anergy.9–12 This led to a search for an immunotherapeutic agent against leprosy which could target the specific anergy towards M. leprae.
Experiments and studies dating back to the 1970s led to the discovery of 5 out of 16 mycobacteria, which caused a blast transformation of T cells from not only tuberculoid leprosy but also LL patients.13 To clinically evaluate the effect of the vaccine on immunity, 32 clinically and histopathologically confirmed treated cases of BL/LL leprosy were administered a single intradermal injection of 5x107 autoclaved Mw.14 They were negative to lepromin testing using Mitsuda and Dharmendra antigen at the start of the study and were adequately treated till bacteriological negativity. On retesting for lepromin reaction 4–6 weeks later, 20 patients (60%) had a positive reaction with both Dharmendra and Mitsuda antigens. Biopsies from all these patients demonstrated mononuclear inflammation, and 12 out of 30 patients showed granuloma formation. A repeat lepromin test at 6–11 months after immunisation revealed persistent positivity.
The utilisation of MIP as an adjunct to Multibacillary (MB)-MDT was initiated in the 1990s. A study conducted in MB leprosy patients demonstrated that the addition of eight doses of MIP to MDT resulted in the conversion of a lepromin test, quicker clinical improvement, rapid clearance of granulomas histologically, a faster drop in Bacillary Index (BI), and earlier release from treatment.15 De Sarkar et al. studied the effects of the MIP vaccine in leprosy patients with BI >2 along with 1 year of MB-MDT.10 The patients in this study received four doses in contrast to the previous eight doses. There was significantly better clinical, bacteriological and histopathological improvement in the study arm compared to the placebo arm. However, lepromin conversion was not a substantial observation. In another similar study, 136 MB patients were administered a combination of MB-MDT and four doses of MIP vaccine 3 monthly for a year.11 While most patients with low baseline BI achieved smear negativity quickly, only 50% of patients with high BI (>4) attained smear negativity in the subsequent 2 years. Patients in the MIP group had a rapid disappearance of granulomas on histopathology. Three of the vaccinated patients with an initial BI of >4 had a relapse. A similar study with borderline leprosy patients (150 in the MIP and MDT group and 120 in the MDT group) demonstrated results akin to the previous studies.16 Another finding was an increase in the epithelioid cell population in this group suggesting a possible immune activation of the macrophages. A study comparing four doses each of MIP or BCG given 3 monthly as immunotherapeutic agents in a group of 60 MB leprosy patients found them to be comparable in terms of clinical improvement, bacterial clearance, and histological resolution of the disease and significantly better than placebo.12 A summary of studies utilising the immunotherapeutic effects of MIP as an adjunct to MDT is provided in Table 1.17-21 To reiterate, as noted in the table, while MIP has been shown to be effective as an adjunct to MDT in multiple studies, there is no uniformity in the number of doses utilised (ranging from two to eight doses) and the interval of administering the injection (3–6 monthly).19 A long-term follow-up study of patients receiving immunotherapy and chemotherapy (WHO MDT) had shown encouraging results like an early achievement of bacteriological clearance, reduction of the effective treatment period by about 40%, and no reactions and/or relapses after 2 years up to 10–12 years of post-treatment follow-up.21 The utilisation of the MIP vaccine with MDT every 6 months in a series of 98 cases of BL resulted in faster clinical and histopathological recovery.19 Clinically, MIP results in faster clearance of skin lesions (decrease in erythema and infiltration of lesions) [Figures 2a and 2b]. The available data shows that MIP is helpful as an adjunct to MDT in MB cases; however, more studies are required to compare the efficacy of the different dosing schedules and their impact on outcomes.
| Study | Sample size | Intervention | Clinical outcome | Histopathological outcome | Bacteriological outcome | Any other outcome | Adverse reaction |
|---|---|---|---|---|---|---|---|
| Talwar et al.17 | 91 (54 + 37) | MIP vaccine or placebo every 3 months | Significant decrease in clinical score | Upgrading seen but statistically non-significant |
Significant decrease in BI (At 12 months of follow up the % decrease in BI was: BB and BL group – 65.6% in the MIP group, 22.48% in the placebo group; LL with BI<4 – 28.49% in the MIP group and 12.23% in BCG group; LL with BI 4–6 + - 8.11% in the MIP group and 6.10% in the BCG group) |
Significant Lepromin conversion (100% vs. 44.4% of BB, 85.7% vs. 7.7% of BL patients, and 61.5% vs. 6.6% of LL converted after four doses of the vaccine vs. placebo, respectively) |
Local erythema, induration, ulcer, scar |
| Zaheer et al.15 | 81 (45 + 36) | 8 doses of MIP vaccine or placebo every 3 months | Significant decrease in clinical score |
Significant upgrading/ clearance |
Significant decrease in BI (At 12 months in the LL group: 1.84 ± 0.18 in the vaccine group and 0.98 ± 0.11 in the group; BL group: 1.64 ± 0.20 in the vaccine group and 0.63 ± 0.14 in the placebo group; BB group: 0.76 ± 0.18 in the vaccine group and 0.46 ± 0.09 in the placebo group) | Significantly shorter treatment duration (13/17 vaccinated LL patients were released from treatment after 2 years in contrast to 2/15 controls) | More type 1 reaction than placebo |
| Sharma et al.18 | 304 (157 + 147) | 8 doses of Mw vaccine or placebo every 3 months | No significant difference in ENL and neuritis | Not studied | Not studied | Non-significant higher incidence of type 1 reaction | |
| De Sarkar et al.10 | 40 (20 + 20) | 4 doses of Mw vaccine or placebo every 3 months | Significant decrease in clinical score | Significant decrease in granuloma fraction | Significant decrease | Lower type 2 reaction | Local ulcer, higher type 1 reaction |
| Kaur et al.11 | 136 | 4 doses of Mw vaccine every 3 months | More rapid fall in BI (54.2% of patients with >4 BI had smear negativity at the end of 3 years while 100% with BI less than 2 had smear negativity at follow-ups of 2 and 3 years) | Local ulcer and scar with regional lymphadenopathy | |||
| Kamal et al.19 | 270 (150 + 120) | Mw vaccine or placebo every 6 months: 2 doses in BT and 5 in BB, BL cases | Significant decrease in clinical score | Significant decrease in granuloma fraction | Significant decrease | Local erythema, induration, ulcer, scar | |
| Narang et al.12 | 60 (20 + 20 + 20) | 4 doses of BCG or MIP or placebo every 3 months | Significant decrease in scores (BCG>MIP) | Significant decrease in granuloma fraction | Significant decrease | Significant decrease in type 2 reaction with BCG | Local erythema, induration, ulcer, scar |
| Katoch et al.20,21 | 36 (12 + 12 + 12) | BCG or MIP or placebo every 6 months | Significant decrease in granuloma fraction | The effective treatment period of achieving bacteriological negativity could be reduced by about 40% faster bacterial clearance. Significantly faster fall in BI in the MIP group than in the BCG or placebo groups; rapid fall in viable bacilli measured by mouse footpad and ATP measurement with BCG and MIP | Time period of reactions reduced by 33%, patients in placebo group continued to have reactions for longer period |
ATP: Adenosine Triphosphate; BB: Mid-borderline leprosy; BCG: Bacillus Calmette Geurin; BI: Bacteriological Index; BL: Borderline leprosy; BT: Borderline Tuberculoid; ENL: Erythema Nodosum Leprosum; MIP: Mycobacterium Indicus Pranii; Mw: Mycobacterium welchii; LL: Lepromatous leprosy.
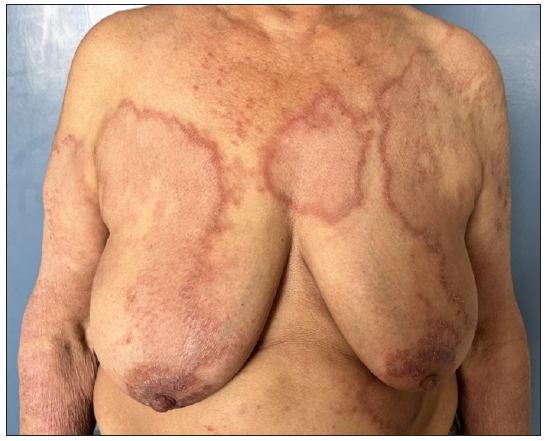
- Case of borderline lepromatous leprosy with multiple large infiltrated and erythematous plaques.
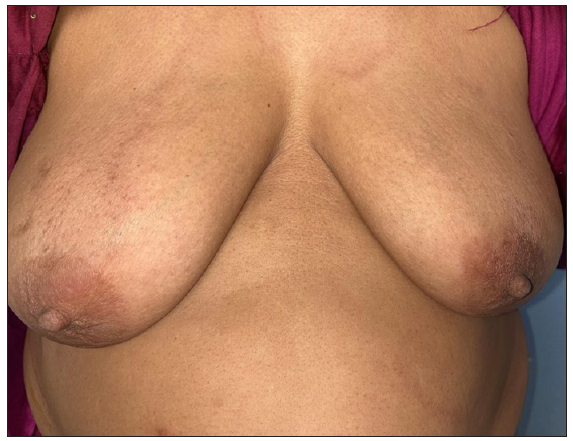
- Decrease in erythema and infiltration after receiving 6 months of multidrug therapy and two doses of MIP vaccine.
Role in immunoprophylaxis
The effect of the vaccine was initially explored in the lepromin conversion of household contacts (HHCs) of MB patients. In a hospital-based study, 192/362 (35.4%) HHCs of MB leprosy patients did not have any evidence of disease and were Mitsuda lepromin negative. After a single dose of the vaccine, lepromin conversion was noted in 82.35% patients and in 98.5% after a second dose. This effect was persistent at a follow-up of 30 months.22
A large trial conducted in South India reported that MIP provided 25.7% protection (CI 1.9–43.8) against leprosy.23 Although lower when compared to the other vaccines, MIP provided statistically significant protection against manifesting disease. The MIP vaccine as an immunoprophylactic agent underwent extensive clinical trials in an endemic region of Uttar Pradesh.18 A total of 24,060 HHCs of 4983 leprosy patients in 272 villages were enlisted. MIP (two doses) were administered to the patients and HHCs 6 months apart. The patients and HHCs were followed up for 10 years and examined for the development of leprosy among the HHCs. An efficacy of 69% was noted at 3–4 years, 59% at 6–8 years, and 39% after 9–10 years of vaccine administration. The vaccination of patients and HHCs did not confer any additive effect compared to the vaccination of HHCs alone, and patient immunisation did not have a protective effect on contacts. A booster dose was advised for the HHCs 7–8 years after the initial dose.
Another study recruited 128 leprosy contacts, out of whom 17 were found to be positive for anti-phenolic glycolipid-1 (anti PGL-1) antibodies.24 They were given one dose of the MIP vaccine. The anti PGL-1 titres were found to decline, and no participant developed leprosy in the follow-up period of 3 years.
Effect on reactional episodes
With standard MDT, bacterial killing and antigen removal can take up to 4–6 years.9,25 MDT targets mainly live bacteria but even dead bacilli and their products can cause immune activation, leading to leprosy reactions. This interrupts the usual indolent course of leprosy, causes neuritis and nerve damage, and adds to the morbidity. It is important to note the alteration of reactional episodes with the use of immunotherapy since reactions are secondary to a sudden increase in the immunity of the patient. In the study by De Sarkar et al., the MIP group had a higher incidence of type 1 reaction (T1R) (30% vs. 10%), while the placebo group showed a higher incidence of type 2 reaction (25% vs. 15%) and reaction associated neuritis (20% vs. 10%).10 Similarly, in another study, the participants had a higher incidence of T1R compared to the placebo arm, but the neuritis/nerve function impairment and incidence of ENL were comparable in the two groups.15 Gupta et al. reported a patient with ENL refractory to steroids, thalidomide, methotrexate and minocycline responded favourably to a single dose of the MIP vaccine.26
In another study, a slightly higher incidence and severity of T1R was demonstrated in the vaccinated group.27 But once the reaction was adequately treated, the recurrence was less frequent, probably due to the gradual improvement in cell-mediated immunity. Notably, there were no additional neurological complications in the vaccine group.
A long-term follow-up study demonstrated similar severity of reactions in the MDT with BCG, MDT with MIP, and MDT with placebo groups but ENL occurred only in the first 2 years in the immunotherapy groups; however, it continued to occur until 3 years in the placebo group.21 Thus, most studies have found MIP to be safe in leprosy, with an overall favourable effect on reactional episodes, particularly ENL.
Rarely, exacerbation of ENL has also been reported with the MIP vaccine.28 A case with multiple ENL episodes, each occurring 10–14 days after administration of MIP was reported; however, the severity of the reaction decreased with each subsequent dose.28
An important consideration in the use of vaccines during lepra reaction is the immunosuppression induced by steroid usage and its impact on the efficacy of the vaccine. Although there are no studies available on the use of MIP in patients receiving steroids, the recent COVID-19 pandemic and the use of the COVID-19 vaccine in varied situations led to the development of a number of guidelines.29 The ideal time for vaccination of patients is one month after discontinuation of the immunosuppressive dose of steroids to elicit an adequate immune response.30 If steroids require prolonged usage, it is better to prescribe the vaccine when receiving the lowest dose of corticosteroid (below 20 mg/day).
Adverse effects
While MIP is a fairly safe vaccine, local injection site reactions are the most common side effects including papule, nodule or plaque formation with or without erythema and/or tenderness16,12,19 [Figure 3]. These lesions may ulcerate and heal with scarring [Figure 4]. Regional lymphadenopathy can also occur.11 Hypertrophic scarring and keloid formation can occur in predisposed patients.18 Very rarely, generalised granulomatous dermatitis has been reported31,32 [Figures 5a and 5b]. Two patients presented with multiple erythematous papules and plaques along with persistent ulceration at the vaccination site. They responded favourably to minocycline with a resolution of lesions within 3 months.31 In another study, a HHC developed an exanthematous rash on the day after vaccination which improved with antihistamines and oral steroids.18 A recent case series reported multiple injection site nodules, one month after administration of the MIP vaccine as a part of treatment for COVID-19.33
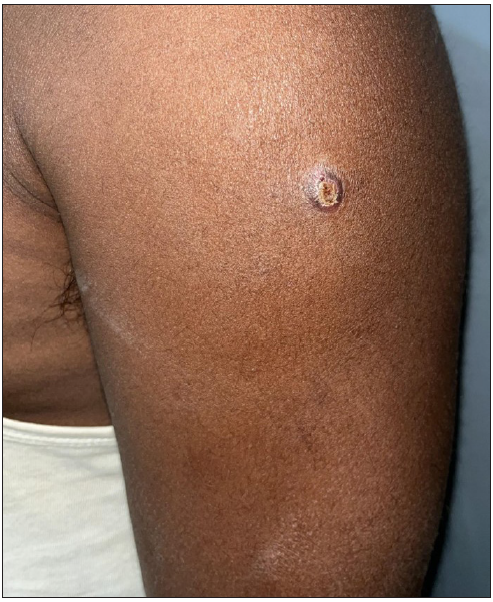
- Nodulo-ulcerative lesion on the left arm – 2 weeks after administration of MIP vaccine.
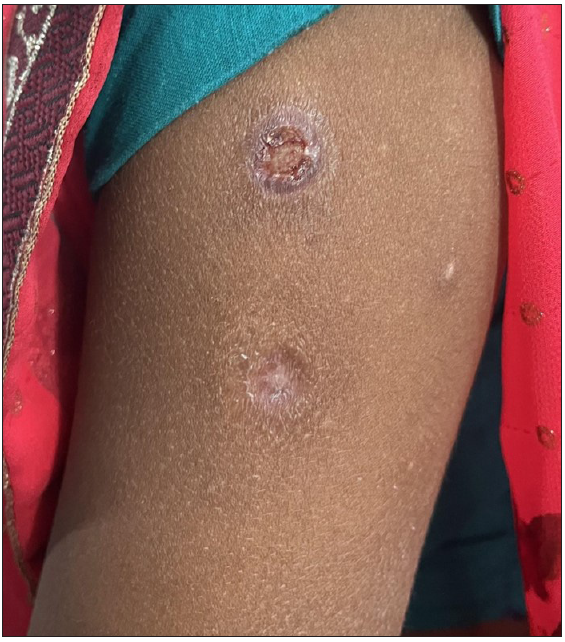
- Superficial ulcer with surrounding erythema on left arm – 1 week after administration of MIP vaccine. Old healed scars can also be noted.
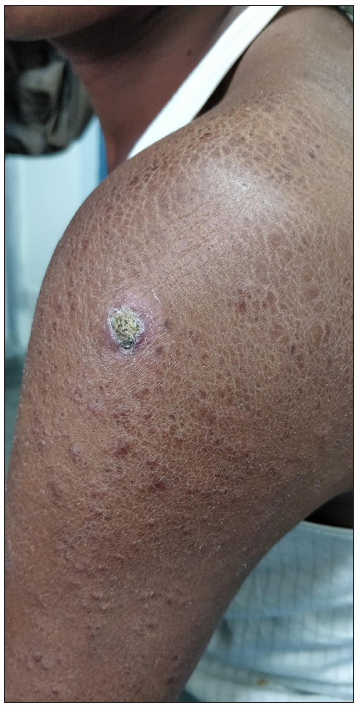
- Patient with granulomatous dermatitis after MIP vaccination showing crusted erythematous nodule at the site of injection surrounded by ichthyosiform changes and multiple reddish-brown papulonodules on the left upper limb.
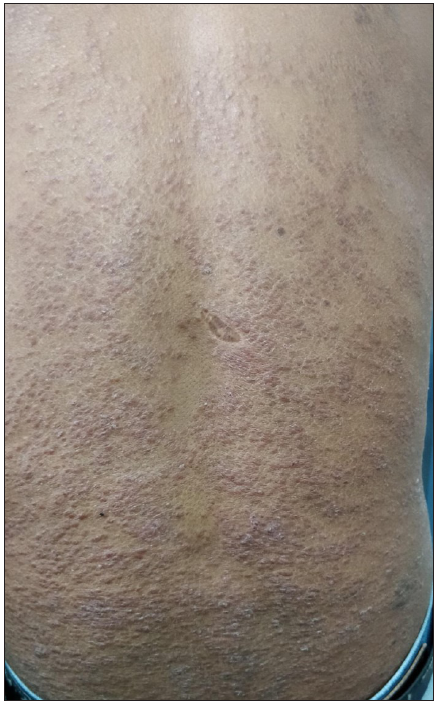
- Patient with granulomatous dermatitis after MIP vaccination showing multiple reddish brown papules with superficial scaling on the back.
There is no defined age cut-off for the use of the MIP vaccine. In the study by Kamal et al., the vaccine was administered safely in paediatric cases.19 There are no studies elucidating the safety of the MIP vaccine in pregnant and lactating women and hence it should be avoided.34
Current recommendations
The Guideline Development Group of WHO has pointed out the moderate quality of evidence for the efficacy of the MIP vaccine in preventing leprosy.35 It is listed as one of the effective vaccines for the prevention of leprosy that could be used under national programmes, with or without chemoprophylaxis, for contact with leprosy patients and the general population. Evidence of adverse effects was found to be limited.
The National Leprosy Eradication Programme of India (NLEP) introduced the MIP vaccine for immunotherapy in the pilot phase in 2016.36 According to the latest 2023–2027 National Strategic Plan and Roadmap for Leprosy, MIP is advocated as an effective and safe immunoprophylactic agent in leprosy and needs to be administered to contacts of index leprosy cases and followed up.37 MIP when combined with MDT helps in faster bacterial clearance, and granuloma clearance, and decreases the incidence and severity of reactions. It has been studied across several tertiary care centers and in the field over the last four decades and is well accepted by the patients. It has also been shown to reduce relapses and reactions after stoppage of MDT. MIP should be used at least in medical colleges/referral institute settings in leprosy-endemic regions of the world for better patient outcomes. By improving therapeutic outcomes and reducing reactions and their severity, disabilities can be prevented which will further reduce the burden and stigma associated with leprosy.
Combining MIP vaccination as immunotherapy to newly detected cases and as immune prophylaxis to their contacts has been found to be a very cost-effective strategy in India. A model-based evaluation of the cost-effectiveness of MIP vaccination in addition to MDT and also for prophylaxis of contacts of leprosy in NLEP was carried out.38 The study estimated an incremental cost-effectiveness ratio of ₹73,790 for each discounted quality-adjusted life year gained by vaccinating both the newly detected case and their contacts (6–20 contacts per index case) over a 5-year period. Significant health gains were, a decreased incidence of new cases, a lesser number of reactions including a reduction in their severity, and prevention of disabilities after release from treatment. The approximate budget for the vaccination was ₹275 million (₹13 million for new leprosy patients and ₹262 million for their contacts) for 5 years. The monetary benefit would be approximately ₹1450 million over a 5-year period. The resulting return on investment was 1.62 times the investment on vaccination which found MIP to be a very cost-effective strategy.34 Currently in India, the MIP vaccine is available as a multidose Sepsivac, 0.6 mL vials by Cadilla Pharmaceuticals which cost ₹7425. It is also available for use as an immunomodulator in certain malignancies including non-small cell lung cancer as a prefilled 0.2 mL syringe (Mycidac-C) at a cost of ₹4060. The cost may be a deterrent factor in the use of the vaccine at an individual level since leprosy mainly affects the lower socio-economic strata. This underscores the necessity of incorporating the vaccine into government policies and making it an integral component of national programmes, ensuring its accessibility to those for whom it is recommended.
Ongoing trials
Currently some trials are ongoing on the role of MIP as immunotherapy in leprosy. These trials aim to evaluate the effectiveness of MIP by assessing factors such as the reduction in viable bacilli load, histopathological upgrading, immunological upgrading, along with clinical improvement.39,40
Future prospects
While the MIP vaccine was originally developed for use in leprosy, majority of studies were done in the earlier years and scarce data is available from the last decade. This is a reflection of the fading interest in its applications probably owing to the elimination of leprosy as a public health problem. It is important to emphasise here that leprosy is still a menace in India, as we contribute approximately 60% of the global leprosy cases.41 MIP vaccine has its application in all aspects of leprosy and it could perhaps be one of the final nails in the coffin of this debilitating disease [Figure 6]. The properties of immunological memory and cost-effectiveness make it a compelling addition to the leprosy prophylaxis and treatment regimens in the NLEP as well as other leprosy programmes across the globe.38
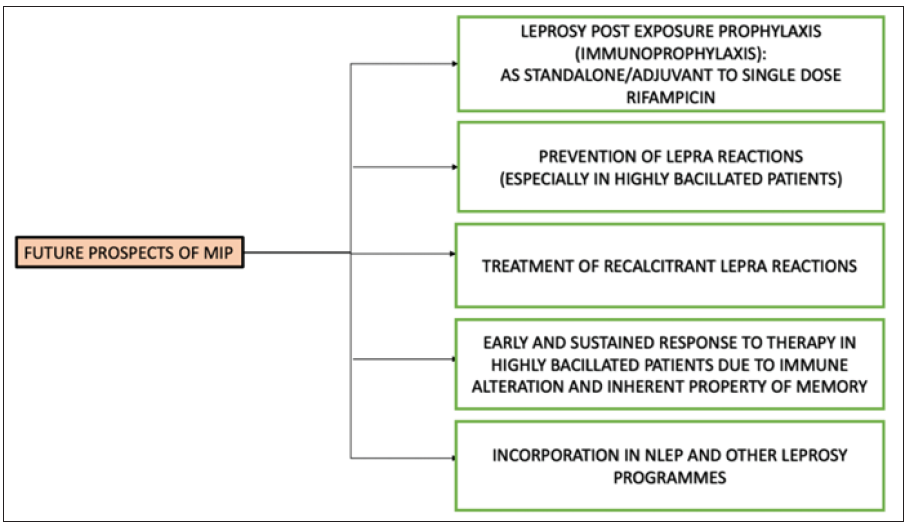
- Schematic representation of the possible prospects of the MIP vaccine. (MIP: Mycobacterium indicus pranii; NLEP: National leprosy eradication programme)
Conclusion
MDT is the standard and effective treatment for leprosy, but immunotherapy has the potential for inducing a longer duration of remission and prevent relapse/reinfection. It is particularly useful in highly bacillated and anergic lepromatous patients where prolonged MB-MDT beyond 1 year is warranted. The vaccine induces rapid and sustained clinical improvement and bacteriological clearance resulting in quicker resolution of disease and eventually lowers the risk of lepra reactions and nerve damage. It is also an effective immunoprophylactic agent for contacts. The vaccine is found to be safe barring minimal local adverse effects and rarely reported systemic side effects. The full potential of this vaccine requires further exploration to allow its use as a routine immunoprophylactic and/or immunotherapeutic agent in leprosy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Strategy 2021–2030; Overview by Erwin Cooreman, Global Leprosy Programme WHO.
- Leprosy vaccines – A voyage unfinished. J Skin Sex Transm Dis. 2021;3:40-5.
- [CrossRef] [Google Scholar]
- Makinga of a highly useful multipurpose vaccine. J Trans Sci. 2016;2:69-73.
- [CrossRef] [Google Scholar]
- Mycobacterium indicus pranii (MIP) vaccine: Pharmacology, indication, dosing schedules, administration, and side effects in clinical practice. Indian Dermatol Online J. 2023;14:753-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human genetic susceptibility of leprosy recurrence. Sci Rep. 2020;10:1284.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics. 2017;11:55-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- T-cell responses to fractionated antigens of Mycobacterium w, a candidate anti-leprosy vaccine, in leprosy patients. Scand J Immunol. 1991;34:23-31.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging concepts of adaptive immunity in leprosy. Front Immunol. 2018;9:604.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Fixed duration multidrug therapy (12 months) in leprosy patients with high bacillary load–Need to look beyond. Indian J Dermatol Venereol Leprol 2023:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of combined Mycobacterium w vaccine and 1 year of MDT on multibacillary leprosy patients. Int J Lepr Other Mycobact Dis. 2001;69:187-94.
- [PubMed] [Google Scholar]
- Combined 12-month WHO/MDT MB regimen and Mycobacterium w. vaccine in multibacillary leprosy: A follow-up of 136 patients. Int J Lepr Other Mycobact Dis. 2002;70:174-81.
- [PubMed] [Google Scholar]
- Comparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2005;73:105-14.
- [PubMed] [Google Scholar]
- Five cultivable mycobacterial strains giving blast transformation and leukocyte migration inhibition of leukocytes analogous to mycobacterium leprae. Lepr India. 1978;50:498-508.
- [PubMed] [Google Scholar]
- Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int J Lepr Other Mycobact Dis. 1983;51:159-68.
- [PubMed] [Google Scholar]
- Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167:401-10.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and histopathological evaluation of the effect of addition of immunotherapy with Mw vaccine to standard chemotherapy in borderline leprosy. Indian J Lepr. 2012;84:287-306.
- [PubMed] [Google Scholar]
- Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine. 1990;8:121-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127-43.
- [PubMed] [Google Scholar]
- Addition of immunotherapy to chemotherapy in pediatric borderline leprosy: A clinical evaluation. Int J Contemp Pediatr 2016:1439-44.
- [CrossRef] [Google Scholar]
- Treatment of bacilliferous BL/LL cases with combined chemotherapy and immunotherapy. Int J Lepr Other Mycobact Dis. 1995;63:202-12.
- [PubMed] [Google Scholar]
- 10–12 years follow-up of highly bacillated BL/LL leprosy patients on combined chemotherapy and immunotherapy. Vaccine. 2004;22:3649-57.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of lepromin positivity by a candidate anti-leprosy vaccine Mycobacterium w in lepromin negative healthy contacts of multibacillary leprosy patients. Indian J Lepr. 1992;64:495-500.
- [PubMed] [Google Scholar]
- Comparative leprosy vaccine trial in south India. Indian J Lepr. 1998;70:369-88.
- [PubMed] [Google Scholar]
- Mycobacterium indicus pranii vaccine immunoprophylaxis in anti-phenolic glycolipid-1-positive leprosy contacts - A pilot study from a tertiary care center in North India. Indian J Dermatol Venereol Leprol. 2021;88:47-50.
- [CrossRef] [PubMed] [Google Scholar]
- Fixed-duration therapy in leprosy: Limitations and opportunities. Indian J Dermatol. 2013;58:93-100.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic recalcitrant erythema nodosum leprosum: Therapeutic dilemma and role of Mycobacterium Indicus Pranii vaccine. An Bras Dermatol. 2022;97:49-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reversal reaction in multibacillary leprosy patients following MDT with and without immunotherapy with a candidate for an antileprosy vaccine, Mycobacterium w. Lepr Rev. 1993;64:219-26.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent erythema nodosum leprosum associated with Mycobacterium indicus pranii vaccine in a case of leprosy: A rare paradox. J Eur Acad Dermatol Venereol. 2021;35:e391-e393.
- [CrossRef] [PubMed] [Google Scholar]
- The COVID-19 vaccine and interventional procedures: Exploring the relationship between steroid administration and subsequent vaccine efficacy. Pain Pract. 2021;21:966-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vaccination recommendations for adult patients with rheumatic diseases. Eur J Rheumatol. 2016;3:29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Generalized granulomatous dermatitis following Mycobacterium w (Mw) immunotherapy in lepromatous leprosy. Dermatol Ther. 2017;30:e12441.
- [CrossRef] [PubMed] [Google Scholar]
- Injection site granuloma due to Sepsivac. Indian Dermatol Online J.. 2024;15:113-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mycobacterium indicus pranii vaccination-induced cutaneous granuloma in COVID-19 patients. Indian Dermatol Online J. 2023;14:908-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of a highly immunogenic recombinant candidate vaccine against human chorionic gonadotropin. Vaccine. 2011;29:2341-8.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the diagnosis, treatment and prevention of leprosy.
- Current situation of leprosy in India and its future implications. Indian Dermatol Online J. 2018;9:83-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- directorate general of health services. ministry of health and family welfare. Government of India; National strategic plan and roadmap for leprosy 2023–2027; 2023 Jan [cited 2023 Sep 12]. Available from: https://dghs.gov.in/WriteReadData/userfiles/file/Leprosy%20New/NSP%2%20Roadmap%20for%20Leprosy%202023-2027.pdf
- Cost-effectiveness of incorporating Mycobacterium indicus pranii vaccine to multidrug therapy in newly diagnosed leprosy cases for better treatment outcomes & immunoprophylaxis in contacts as leprosy control measures for National Leprosy Eradication Programme in India. Indian J Med Res. 2021;154:121-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- New Delhi: database publisher (India). 2020 Nov 2 - . Identifier CTRI/2020/11/028803, A prospective study to evaluate the bacteriological and antigen-specific immunological responses induced by MIP and/ BCG vaccines as adjunctive treatment (immunotherapy) in multibacillary leprosy patients treated with multidrug therapy; 2020 Oct 20 [cited 2023 Sep 09]; [1 page]. Available from: https://ctri.nic.in/Clinicaltrials/showallp.php?mid1 = 48275&EncHid = 94873.74757&userName = mip%20vaccine
- New Delhi: database publisher (India). 2021 Sep 8 - . Identifier CTRI/2021/09/036335, An open label assessor-blind, phase III multi-centric, randomized controlled study trial to evaluate the immunotherapeutic efficacy of Mycobacterium indicus pranii (MIP) vaccine, Bacillus calmette guerin (BCG) vaccine as an adjunct to chemotherapy in multibacillary (MB) patients of leprosy; 2021 Sep 6 [cited 2023 Sep 09]; [1 page]. Available from: https://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid = 45941&EncHid = 94873.74757&userName =
- Weekly Epidemiological Record, 2023, vol. 98, 37 [full issue] Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire. 2023;98:185-94.
- [Google Scholar]







