Translate this page into:
Mongolian spots
Correspondence Address:
Devinder Mohan Thappa
Department of Dermatology and Sexually Transmitted Diseases, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry-605 006
India
| How to cite this article: Gupta D, Thappa DM. Mongolian spots. Indian J Dermatol Venereol Leprol 2013;79:469-478 |
Abstract
Mongolian spots (MS) are birthmarks that are present at birth and their most common location is sacrococcygeal or lumbar area. Lesions may be single or multiple and usually involve < 5% total body surface area. They are macular and round, oval or irregular in shape. The color varies from blue to greenish, gray, black or a combination of any of the above. The size varies from few to more than 20 centimetres. Pigmentation is most intense at the age of one year and gradually fades thereafter. It is rarely seen after the age of 6 years. Aberrant MS over occiput, temple, mandibular area, shoulders and limbs may be confused with other dermal melanocytoses and bruises secondary to child abuse, thus necessitating documentation at birth. Although regarded as benign, recent data suggest that MS may be associated with inborn errors of metabolism and neurocristopathies. Mongolian spots usually resolve by early childhood and hence no treatment is generally needed if they are located in the sacral area. However, sometimes it may be required for extrasacral lesions for cosmesis.Introduction
Mongolian spot is a type of dermal melanocytosis, which presents at birth as an ill-defined area of slate gray to blue black pigmentation over the lumbosacral region, and disappears during childhood.
Historical and Anthropological Perspective: Facts and Fiction
Through the centuries, Mongolian spots (MS) have been the subject of many stories and controversies, both biologically and anthropologically. Earliest known accounts of MS date back to Hippocrates, who believed that a blow to the pregnant mother′s abdomen manifests as a mark at the corresponding place in the newborn. [1]
A similar theory also prevailed in Turkey, where it was known as ′leke′ or ′spot′. [2] Father Gumilla was the first Westerner to describe MS in his writings. [1] The Mongolian spot is referred to in Japanese asshirigaaoi, meaning to have a blue bottom, and is believed to be the consequence of coitus performed during pregnancy, or a mark made by the gods presiding over births. [3] In China, it is known simply as ′mark′ (taiji), where, when a child is born, God gives it a ′spank′ to give it a start in life. According to one legend, the ′King of the Underworld′ slaps the child to make it come out. [2] This is reflected in the Mexican term for MS, ′la patada de Cuahutemoc′, meaning Cuahutemoc′s kick. [3] A similar myth prevails in Kyrgyzstan, where Umaiene (′Heavenly Mother′), the patron of infants, gently slaps the child while it is still in the womb. According to Armenian superstition, if a woman works on certain days or if another woman criticizes her and places her hand on the abdomen, then the child is born with this mark or ibid at that spot. [2]
It was a German professor Edwin Baelz who, in 1885, described it in Mongolians and named it ′Mongolen Flecke′ or Mongolian spot. Baelz believed that MS was a distinct characteristic of the Mongols and other non-Caucasian races. [1] During late 19 th and early 20 th century, Mongolian spots were the topic of many anthropological debates and research papers. Some scientists believed that presence of MS in Mongols, Japanese, Chinese, Turks, Koreans, Hungarians etc. reflected a common Central Asian origin of these races whereas others in Mongolia believed that MS in other populations was a legacy of the invading armies of Huns and Mongols, thus "implicating Mongolia to be the cradle of the Eurasian civilization". [2] According to Cordova, Baelz′s theory of exclusivity of MS to Mongoloid races was opposed by Adachi and El Bahrawy, who opined that it was also found in Europeans and other small groups like the Native American Sioux, Inuits, Samoans and other Polynesians, who had no connection with the Mongoloid race. [1] Another interesting theory states that a genetic mutation occurred in the Mongolians about 10,000 years ago, which resulted in MS, and which can be used to track movements of the human population to as far as Greenland and North and Central America. [2]
Ashmead proposed that MS first originated in Negroes and then spread to Asia and Europe. According to Ratsimamanga, MS represented an atavistic rudimentary formation of the simian tail. Larsen and Godfrey formulated a Mendelian theory of inheritance for the MS. [1]
Epidemiology
Both sexes are equally affected. A unique feature of MS is the varying prevalence based on ethnicity of the population. They are most common in Asians and Africans, and less common in Caucasians. [1] In different studies, the frequency was found to be 25% in Australians, 7% in Jews, 12% in Arabs and 13-25% in Turkish babies. [4],[5],[6],[7] It was seen in 75% of Nigerians and 50% of Brazilians. [8],[9] The frequency varied from 10-70% in Iranian neonates. [10],[11] It was 80% in the Japanese, 62% in the Taiwanese and 86-100% in the Chinese newborns. [12],[13],[14],[15] Figures from India also reflect the impact of ethnicity. Two studies conducted in Punjab and Chandigarh (North India) found the frequency of MS to be 60% and 62% respectively. [16],[17] On the other hand, three studies conducted in Karnataka (South India) on cutaneous lesions in the newborns found the frequency of MS to be 69%, 72% and 89% respectively, whereas a similar study from Pondicherry (South India) found it to be 78%. [18],[19],[20],[21]
Some of the large detailed studies done on MS are described below:
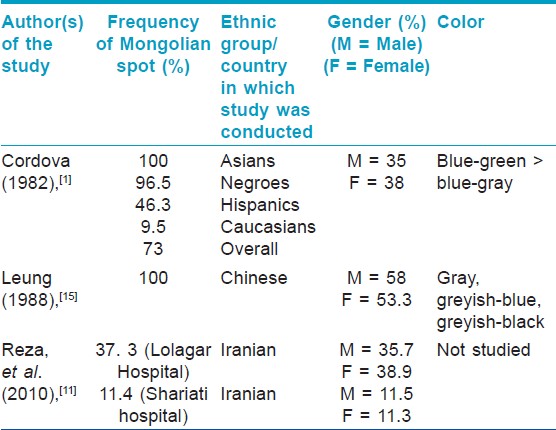
In the Cordova study (1982), [1] conducted in USA, 437 consecutively born full term neonates were examined for the presence of MS. In this, 250 out of 259 Blacks (96.5%), 62 out of 134 Hispanics (46.3%), 4 out of 42 Caucasians (9.5%) and both the Asian babies (100%) were found to have the spot. The overall frequency was 73% (318/437), of which, 35% males and 38% females had MS. Sacrogluteal region was the most common location. Upper limbs and extensors were more commonly involved than lower limbs and flexors. MS were not seen over the trunk, palms or soles. Most of the MS were irregular and the most common color was blue-green.
Leung (1988), [15] studied 92 Chinese Canadian new born infants (49 boys and 43 girls) and 1633 Chinese Canadian children (819 boys and 814 girls) and found that MS were present in all newborns and disappeared slowly until 6 years of age when the rate of disappearance increased. The overall incidence was 58% in boys and 53% in girls. The most frequent site of involvement was the sacrococcygeal area and both sides of body were equally affected. No spots were found on the face, neck, perianal area, palms, or soles. The color varied from greyish blue to greyish black. Reza, et al., (2010), [11] studied 2305 consecutive newborns at Shariati hospital and 1706 at Lolagar hospital, Iran for 2 years. In Shariati hospital, MS was observed in 262 neonates (11.4%). The lesions were found most commonly at the sacral area (85.1%). They were seen in 11.5% boys and 11.3% girls.
In Lolagar hospital, MS was observed in 637 neonates (37.3%). The lesions were mostly found in the sacral area (80.8%). They were seen in 35.7% of boys and 38.9% of girls.
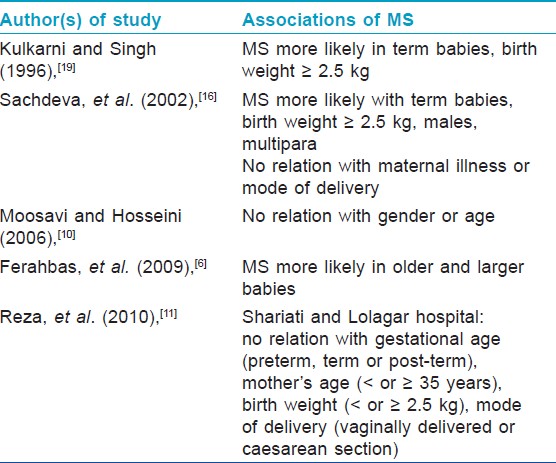
The salient features of the above three studies are summarized in [Table - 1] and [Table - 2]. Finding of [Table - 3] describes associations of MS found by various authors in different studies.

Kikuchi hypothesized that dermal melanocytes were found in the buttocks of all the newborns irrespective of race. However, some babies had more number of melanocytes as compared to others, and this manifested clinically as a slate blue colour over the buttocks. Also melanocytes in the Whites contained inactive, incompletely melanised melanosomes. Duration of dermal melanocyte production, which was more in Asians as compared to Caucasians, was also proposed to play a role. [22]
Pathogenesis
Microscopically, dermal melanocytes have been found in the foetus by the age of 3 months and macroscopically by the age of 7 months. [22] Melanocytes are derived from melanoblasts that arise from neural crest cells and migrate dorsolaterally between the mesodermal and the ectodermal layers to reach the basal layer of epidermis and hair follicle. Melanocytes are present in dermis of the embryos, at the beginning of 10th week of gestation, and they migrate to the epidermis between 11 th -14 th week. After 20th week, no melanocytes are found in the dermis. [23] This is attributed both to the migration of melanocytes to epidermis and their clearance by macrophages. [24] Failure of these mechanisms results in MS.
Mechanism of regression vs. Persistence of MS
The natural disappearance of the Mongolian spots is a unique phenomenon hitherto not observed in other dermal pigmentary conditions. Electron microscopy shows that dermal melanocytes are enclosed by a protective extracellular fibrous sheath. Fading Mongolian spots gradually lose this sheath and undergo destruction, whereas it is preserved in persistent MS. This destruction starts as early as during foetal life and becomes most intense during early childhood. [22]
Other postulated mechanisms for the persistence of dermal melanocytes include local abundance of melanocyte stimulating growth factors, defective regulation of melanocyte proliferation in the epidermal melanin unit and genetic factors. [25]
Earlier Theories Regarding the Natural History of The Mongolian Spot
Inoue divided the natural history of MS into two stages: Stage of evolution is characterised by a positive DOPA reaction whereas stage of regression is characterised by a negative DOPA reaction. [22]
The Local Hindrance Phenomenon
According to this, the migration of Mongolian spot cells early in foetal life is affected by the geography of its route. It was noted that the MS never involves the perianal area, just like nevus of Ota never extends beyond the nasolabial fold. This was attributed to the local geography preventing the Mongolian spot cells from entering that area. [22]
It was also believed that since dermal melanocytes are interspersed between collagen bundles, proliferation of collagen after one year of age results in compression and destruction of melanocytes. Another theory was that persistence of MS was due to intimate contact of dermal melanocytes with neighbouring blood vessels in certain individuals. [22]
Why Are Mongolian Spots Blue?
The blue color of Mongolian spots is secondary to the Tyndall effect, whereby, the longer- wavelength light is more transmitted while the shorter-wavelength light is more reflected via scattering. Dermal pigmentation appears gray, greyish-blue or greyish black because these colors have a shorter wavelength and are reflected to the skin surface. The amount of melanin in the dermal melanocytes, the number of dermal melanocytes and their depth in the dermis are also important determinants of color. [22],[24]
Pathology
Histology
In low magnification, mid-lower dermis shows elongated, spindle shaped, bipolar, wavy or irregular, dendritic cells, 5-10 μm thick and 30-100 μm in length, lying parallel to the skin surface without disturbing the normal architecture of skin. At high magnification, these have been shown to contain melanin granules, stained positive with Masson-Fontana silver stain. [26] The centre of the cell is the widest portion, containing a pale, oval, lightly stained nucleus. The cells may have long processes and may be slender or pear-shaped. [1] On the other hand, unaffected skin showed sparse dermal melanocytes with incompletely melanized melanosomes. [26]
Immunohistochemistry
These cells stain positively with S-100, HMB45, Melan A/MART-1, Tyrosinase, PNL-2 Ag and MITF. [27]
Ultra structure
On electron microscopy, dermal melanocytes have been shown to contain abundant melanosomes. They are also invested by a patchy extracellular sheath which is highly developed in persistent MS and, is made up of both fine granules and filaments. This external lamina runs parallel to the cell membrane of the melanocyte at a distance of 20-50 nm from it. The maximum width of the sheath is 1.5 μm. It shows a marked decline with age. [28]
Clinical Features
Mongolian spots are present at birth or appear soon thereafter, although late onset has been described. Carmichael, et al., described a 26 year old Asian male with a 6 year history of a macular patch of diffuse grey-blue hyperpigmentation centred on the left loin, extending beyond the mid-line posteriorly and onto the lateral abdominal wall anteriorly. [27] The most common location is sacrococcygeal or lumbar area. Aberrant MS over occiput, temporal and mandibular areas may actually be examples of nevus of Ota. [29],[30] Lesions may be single or multiple. In their study, Leung, et al., found that 65.5% cases had less than 5% total body surface area (TBSA) involvement, 28.9% cases had 5-10% TBSA involvement, and only 5.6% had greater than 15% total body surface area (TBSA) involvement. [15]
They are macular and round, oval or irregular in shape. The color varies from blue to greenish, gray, black or a combination of any of the above. The size varies from few to more than 20 cm. Pigmentation is most intense at the age of one year and gradually fades thereafter. [22] It is rarely seen after the age of 6 years. [15]
However, persistent MS have been described by Hidano, [31] and Kikuchi, [32] in adult Japanese males with a frequency of 4.1% and 2.88 % respectively. Persistent MS have also been described at extra-gluteal sites in adult Chinese women. [23]
The MS are most commonly classified as sacral and extra-sacral. Based on the speed of regression, they have been classified into three further types. [22]
- Common type- these regress normally by early childhood
- Extensive type- these regress very slowly
- Persistent type- these may persist into adulthood
Other variants include the following types:
- Generalized MS: Involve large areas covering almost entire anterior or posterior trunk and extremities [Figure - 1].
- Aberrant MS: Involve unusual sites such as head and neck region or extremities [Figure - 2] and [Figure - 3]. [1]
- Superimposed MS: A darker Mongolian spot overlies a lighter one. [24]
- Halo-like MS: When café-au-lait macules and melanocytic nevi reside within areas of Mongolian spot, there will be a white rim lacking dermal melanocytes surrounding each lesion. [33]
- Speckled MS: Present as groups of dotted pigmentation. [31]
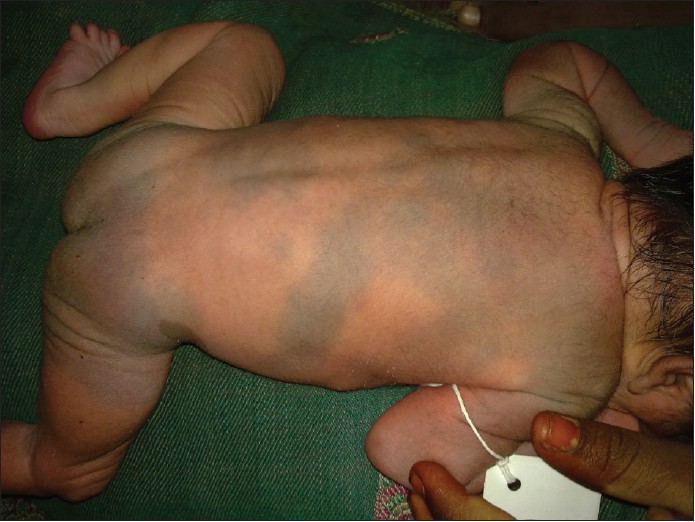 |
| Figure 1: Multiple Mongolian spots with café-au-lait macule in a normal baby |
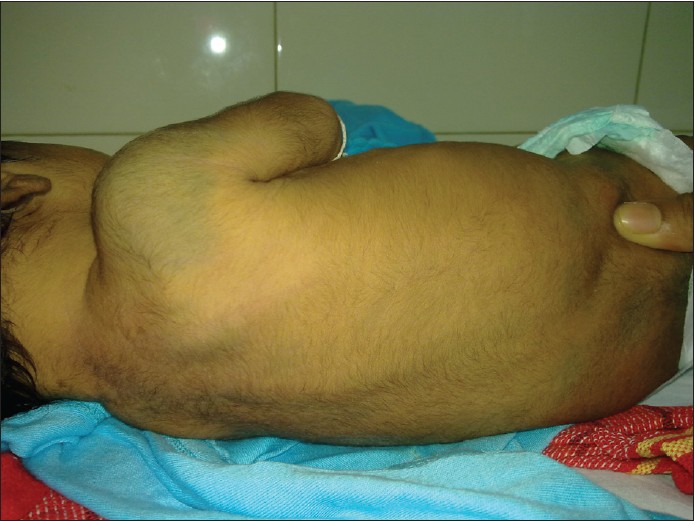 |
| Figure 2: Aberrant locations of Mongolian spot over shoulder |
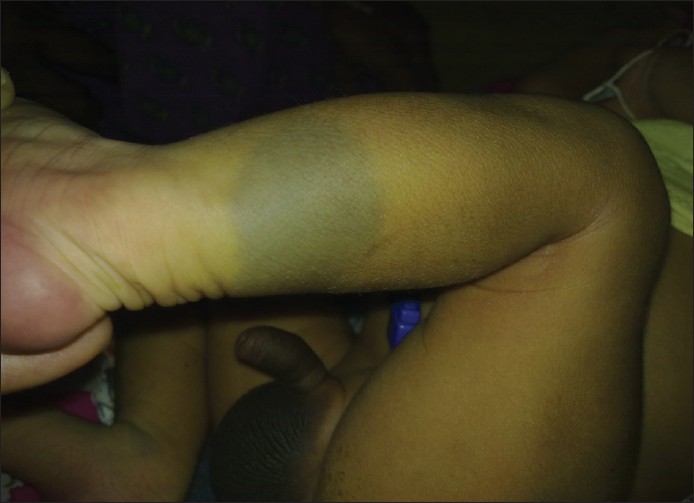 |
| Figure 3: Aberrant locations of Mongolian spot over leg |
Differential Diagnosis
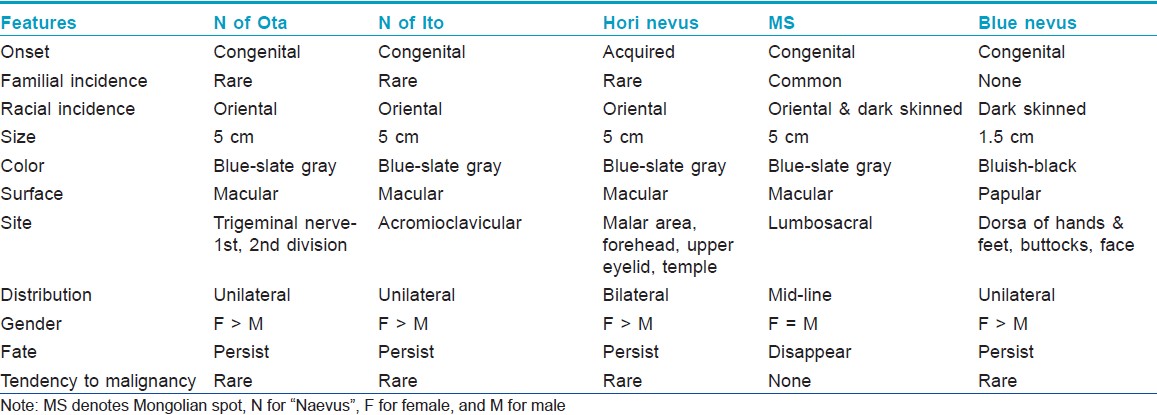
Mongolian spots must be differentiated from other dermal melanocytoses like nevus of Ota, nevus of Ito, Hori nevus and blue nevus. These must be differentiated clinically based on their onset, distribution and evolution [Table - 4], as histopathology in all these conditions is similar. Sometimes they may be confused with aberrant MS. The salient features that help us make a diagnosis of MS are: Onset at birth, disappearance with age, and absence of mucosal involvement/progression to malignancy.
Associations of The Mongolian Spot
Historically, MS have been regarded as benign but recent data suggest that MS may be associated with inborn errors of metabolism and neurocristopathies. Neurocristopathy refers to a disorder characterised by abnormalities in neural crest migration. Examples include dermal melanocytoses, cleft lip and palate, phakomatosis pigmentovascularis and neurofibromatosis. A close relationship between central nervous system and melanocyte population, due to their common origin from neural crest is well known. This explains why these conditions can occur together. [26]
Inborn errors of metabolism (IEMs)
Inborn errors of metabolism arise from single gene defect, most often involving an enzyme function, which leads to disruption of a specific metabolic pathway giving rise to abnormalities in the synthesis or catabolism of proteins, fats or carbohydrates. The most common condition associated with MS is Hurler′s disease [Figure - 4] and [Figure - 5] followed by GM1 gangliosidosis 1. [26] Given the high prevalence of MS in Asians and Africans, the association of these two conditions may be a chance occurrence, but over the last thirty years numerous studies have been published linking the two. In the largest retrospective analysis, 52 Japanese males with Hunter′s disease were examined for the presence, distribution, and colour of Mongolian spots. As a control, 21 brothers of the patients were taken. The overall incidence of extensive MS irrespective of age was 78%. They were all deep blue in color, and disappeared extremely later in their life. All of the brothers who did not have Hunter syndrome had common-type MS, which regressed during their childhood. [34] MS have also been reported in association with mucolipidosis, Niemann-Pick disease and mannosidosis. [34],[35],[36],[37] A similar association has been recently reported from India too. [38]
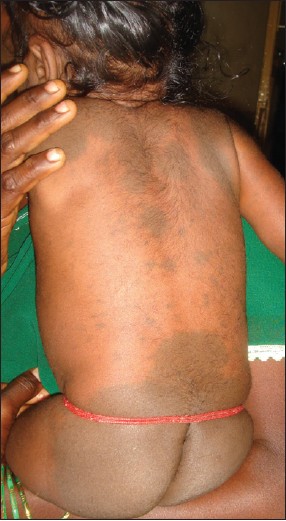 |
| Figure 4: Multiple Mongolian spots over the back in Hurler's syndrome |
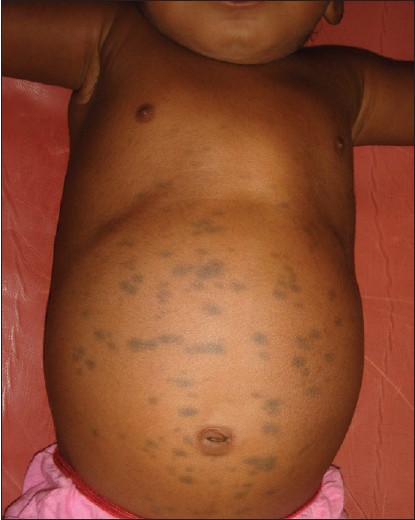 |
| Figure 5: Multiple Mongolian spots over the abdomen in Hurler's syndrome |
Hanson, et al., reviewed 15 reports describing association of dermal melanocytosis with lysosomal storage diseases, with 39 individual cases. Of this, 21 cases were Venezuelans, 5 were African Americans, 4 were Asians, 3 were Europeans, and the data on ethinicity was not available in the remaining 6 cases. [26] These figures again show increased prevalence of extensive MS with IEMs in Asians and Africans as compared to Caucasians.
Proposed mechanism of development of MS in IEMs
Human keratinocytes and dermal fibroblasts express nerve growth factor (NGF) which is important for transdermal melanocyte migration. NGF exerts its action via the Trk protein, a tyrosine kinase-type receptor, which is also present on melanocytes. In IEMs, accumulated metabolites (e.g. GM1 ganglioside in GM1 gangliosidosis and heparin sulphate in Hurler′s disease) bind to the Trk protein resulting in an abnormal increase in NGF activity. This leads to the development of large neural processes and the neurological abnormalities seen in lysosomal storage disease. Since melanocytes also have receptors for NGF, metabolite-Trk binding leads to abnormal melanocyte migration and also triggers melanin synthesis in dormant melanocytes. [26] It may be exciting to think that in Whites, where under normal circumstances melanocytes are inactive, the abnormal metabolites may act as a stimulus for activation of melanocytes.
Pathology of MS in IEMs
Dermal melanocytes in lysosomal storage disorders contain empty lysosomal vacuoles that do not stain with Periodic acid-Schiff, Alcian blue or Giemsa stain. [26],[36]
Clinical presentation of MS in IEMs
MS in IEMs are deeper in color and have a generalized distribution involving dorsal and ventral trunk in addition to sacral region and extremities. They are persistent and in some cases an indistinct feathery border has been described. [26]
Significance of MS in IEMs
Although amniocentesis and chorionic villus sampling can be used to diagnose IEMs prenatally, they are invasive and cannot be used as routine procedures. Semi-quantitative urine spot test for glycosaminoglycans is fraught with the problem of false positives and negatives. [39] However, extensive MS as a marker for IEMs, if used in conjunction with the urine spot test, may improve the sensitivity of early diagnosis. If both are present, enzyme assays can be undertaken for confirmation and this maybe a simple and early screening algorithm. Mucopolysaccharidoses respond well to stem cell transplantation or enzyme replacement therapy if instituted at an early stage, before irreversible organ damage occurs.
Cleft lip
Pigmented macules in the labial skin on either side of a cleft lip have been described which show evidence of dermal melanocytes upon histopathological examination. As these lesions are analogous to Mongolian spots, they have been termed cleft lip Mongolian spot. [40]
Vascular birthmarks
Widespread, persistent and aberrant naevus flammeus co-existing with pigmentary abnormalities like MS and nevus spilus has been termed as phakomatosis pigmentovascularis (PPV). [41]
Classification of PPV is described below. [41] Types II and IV are associated with MS.
Type I Naevus flammeus plus nevus pigmentosus et verrucosus
Type II Naevus flammeus plus Mongolian spots with or without naevus anemicus
Type III Naevus flammeus plus naevus spilus with or without naevus anemicus
Type IV Naevus flammeus plus Mongolian spots and naevus spilus with or without nevus anemicus
Each condition is further divided into type a and b, denoting a co-existing cutaneous and systemic disease respectively.
PPV is due to twin-spotting which involves mosaicism and allelic mutation, one for pigmented lesions and one for vascular lesions with some crossing over during mitotic recombination. This results in homozygous cell populations in different body areas which lead to Mongolian spots and nevus flammeus. The above defect may also be caused by abnormal neural crest migration of melanocytes and angiogenic cells adversely affecting each other. [42]
Mongolian spots have been described in association with non-involuting congenital hemangioma, Sturge-Weber syndrome, Klippel-Trenaunay syndrome, cutis marmorata telangiectatica congenita and segmental café-au-lait macules. [43],[44],[45] In these cases persistent Mongolian spots carry a worse prognosis and may be associated with underlying neurological defects. [42]
Child abuse
In recent years, documentation of the Mongolian spots has assumed medico-legal importance as they can sometimes be confused with bruises, especially if present over atypical sites. This leads to a mistaken diagnosis of child abuse or battered child syndrome. MS can be distinguished from a bruise in that it is not tender, does not change color or evolve with time and may take several months to disappear. [46]
Miscellaneous
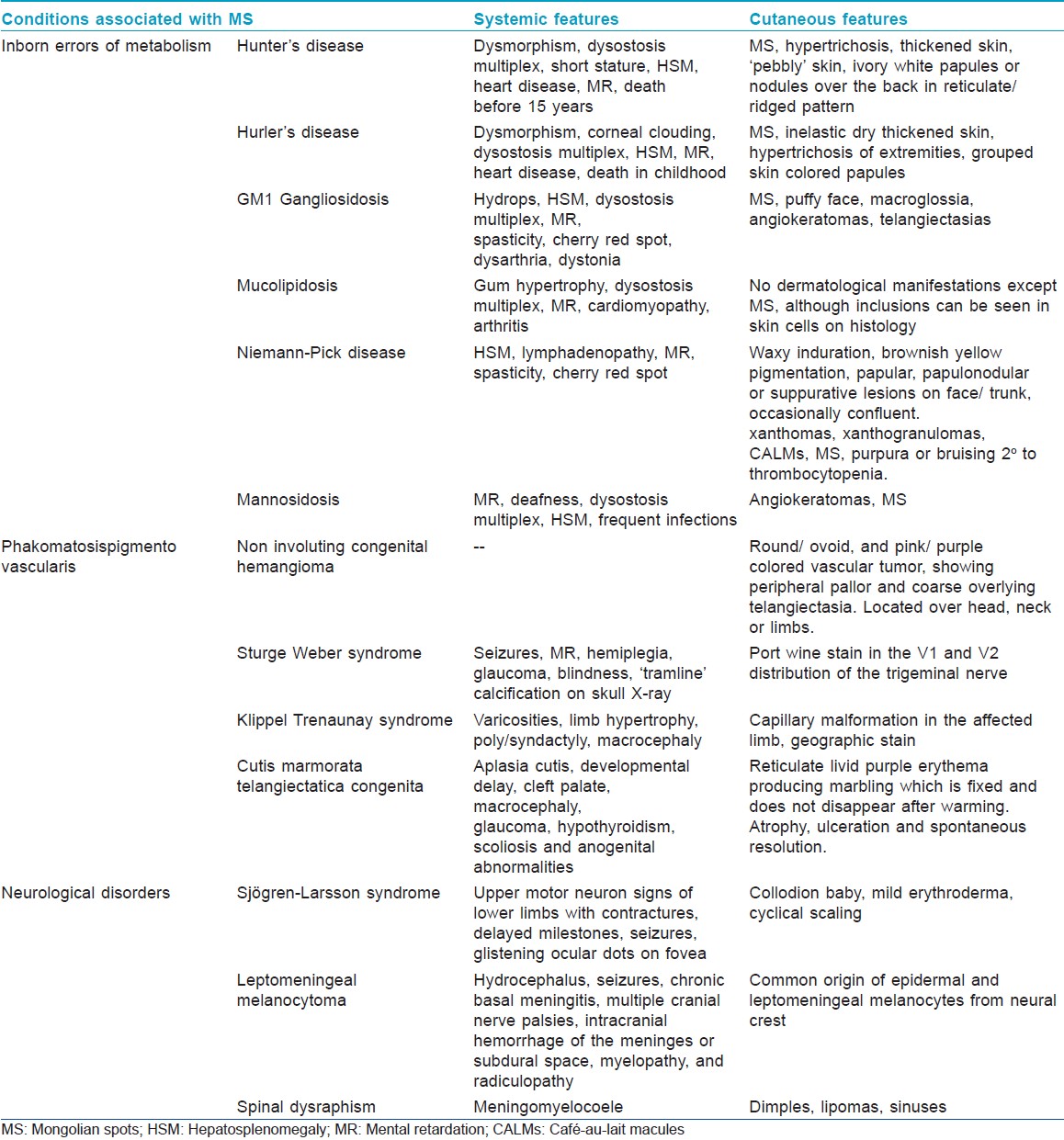
Mongolian spots have been reported to occur with Sjφgren-Larsson syndrome and leptomeningeal melanocytoma involving the spinal cord. [47],[48] They may also represent an occult marker of spinal dysraphism. [49] Observation of [Table - 5] describes the salient features of various syndromes associated with MS.
Treatment
Mongolian spots usually resolve by early childhood and hence no treatment is generally needed if they are located in the sacral area. However sometimes it may be required for extrasacral lesions for cosmesis. Apart from cosmetic camouflage, 755 nm Q-switched alexandrite lasers have been used with a pulse width of 50 ns, treatment dosage of 5.5 J/cm 2 , and 4 mm spot size. [50] Q-switched ruby and Q-switched Nd-YAG lasers are other options. Good results are obtained if treatment is initiated before the age of 20 years. [51]
Conclusion
MS may represent a step in the evolution or in the retrogression of human pigmentary system. Although a lot has been written about Mongolian spots, recent studies showing the association between extensive MS and inborn errors of metabolism point out that the story of Mongolian spots is far from over. Small, light blue-green colored spots confined to lumbosacral area can be ignored, but extrasacral, extensive, persistent and dark colored spots should be looked upon with suspicion, especially in the presence of a consanguineous marriage or a strong family history of storage disorders. Future research should focus on further quantifying and validating parameters like size, %TBSA, location and color of MS, as markers for IEMs and their place in screening and diagnosis of these syndromes.
| 1. |
Cordova A. The Mongolian spot: A study of ethnic differences and a literature review. Clin Pediatr (Phila) 1981;20:714-9.
[Google Scholar]
|
| 2. |
Bille F. The Mongolian blue spot: Symbolism, limitations, potentialities. Paper presented at the conference: Study of Mongolian Symbolism: Quest and Perspectives Ulaanbaatar, Mongolia; 2009 September 09-10.
[Google Scholar]
|
| 3. |
Mongolian spot. Available from: http://en.wikipedia.org/wiki/Mongolian_spot.[Last accessed on 2012 July 12].
[Google Scholar]
|
| 4. |
Rivers JK, Frederiksen PC, Dibdin C. A prevalence study of dermatoses in the Australian neonate. J Am Acad Dermatol 1990;23:77-81.
[Google Scholar]
|
| 5. |
Kahana M, Feldman M, Abudi Z, Yurman S. The incidence of birthmarks in Israeli neonates. Int J Dermatol 1995;34:704-6.
[Google Scholar]
|
| 6. |
Ferahbas A, Utas S, Akcakus M, Gunes T, Mistik S. Prevalence of cutaneous findings in hospitalized neonates: A prospective observational study. Pediatr Dermatol 2009;26:139-42.
[Google Scholar]
|
| 7. |
Egemen A, Ikizoðlu T, Ergör S, Mete Asar G, Yilmaz O. Frequency and characteristics of mongolian spots among Turkish children in Aegean region. Turk J Pediatr 2006;48:232-6.
[Google Scholar]
|
| 8. |
Onayemi O, Adejuyigbe EA, Torimiro SE, Oyelami O, Jegede OA. Prevalence of Mongolian spots in Nigerian children in Ile-Ife, Nigeria. Niger J Med 2001;10:121-3.
[Google Scholar]
|
| 9. |
Zagne V, Fernandes NC. Dermatoses in the first 72 hours of life: A clinical and statistical survey. Indian J Dermatol Venereol Leprol 2011;77:470-6.
[Google Scholar]
|
| 10. |
Moosavi Z, Hosseini T. One-year survey of cutaneous lesions in 1000 consecutive Iranian newborns. Pediatr Dermatol 2006;23:61-3.
[Google Scholar]
|
| 11. |
Reza AM, Farahnaz GZ, Hamideh S, Alinaghi SA, Saeed Z, Mostafa H. Incidence of Mongolian spots and its common sites at two university hospitals in Tehran, Iran. Pediatr Dermatol 2010;27:397-8.
[Google Scholar]
|
| 12. |
Hidano A, Purwoko R, Jitsukawa K. Statistical survey of skin changes in Japanese neonates. Pediatr Dermatol 1986;3:140-4.
[Google Scholar]
|
| 13. |
Tsai FJ, Tsai CH. Birthmarks and congenital skin lesions in Chinese newborns. J Formos Med Assoc 1993;92:838-41.
[Google Scholar]
|
| 14. |
Shih IH, Lin JY, Chen CH, Hong HS. A birthmark survey in 500 newborns: Clinical observation in two northern Taiwan medical centre nurseries. Chang Gung Med J 2007;30:220-5.
[Google Scholar]
|
| 15. |
Leung AK. Mongolian spots in Chinese children. Int J Dermatol 1988;27:106-8.
[Google Scholar]
|
| 16. |
Sachdeva M, Kaur S, Nagpal M, Dewan SP. Cutaneous lesions in new born. Indian J Dermatol Venereol Leprol 2002;68:334-7.
[Google Scholar]
|
| 17. |
Nanda A, Kaur S, Bhakoo ON, Dhall K. Survey of cutaneous lesions in Indian newborns. Pediatr Dermatol 1989;6:39-42.
[Google Scholar]
|
| 18. |
Nobby B, Chakraborty N. Cutaneous manifestations in the new born. Indian J Dermatol Venereol Leprol 1992;58:69-72.
[Google Scholar]
|
| 19. |
Kulkarni ML, Singh R. Normal variants of skin in neonates. Indian J Dermatol Venereol Leprol 1996;62:83-6.
[Google Scholar]
|
| 20. |
Dash K, Grover S, Radhakrishnan S, Vani M. Clinico epidemiological study of cutaneous manifestations in the neonate. Indian J Dermatol Venereol Leprol 2000;66:26-8.
[Google Scholar]
|
| 21. |
Baruah CM, Bhat V, Bhargava R, Garg RB, Ku. Prevalence of dermatoses in the neonates in Pondichery. Indian J Dermatol Venereol Leprol 1991;57:25-8.
[Google Scholar]
|
| 22. |
Kikuchi I. What is a Mongolian spot? Int J Dermatol 1982;21:131-3.
[Google Scholar]
|
| 23. |
Leung AK, Kao CP, Leung AA. Persistent Mongolian spots in Chinese adults. Int J Dermatol 2005;44:43-5.
[Google Scholar]
|
| 24. |
Leung AK, Robson WL. Superimposed Mongolian spots. Pediatr Dermatol 2008;25:233-5.
[Google Scholar]
|
| 25. |
Park JM, Tsao H, Tsao S. Acquired bilateral nevus of Ota-like macules (Hori nevus): Etiologic and therapeutic considerations. J Am Acad Dermatol 2009;61:88-93.
[Google Scholar]
|
| 26. |
Hanson M, Lupski JR, Hicks J, Metry D. Association of dermal melanocytosis with lysosomal storage disease: Clinical features and hypotheses regarding pathogenesis. Arch Dermatol 2003;139:916-20.
[Google Scholar]
|
| 27. |
Carmichael AJ, Tan CY, Abraham SM. Adult onset Mongolian spot. Clin Exp Dermatol 1993;18:72-4.
[Google Scholar]
|
| 28. |
Okawa Y, Yokota R, Yamauchi A. On the extracellular sheath of dermal melanocytes in nevus fusco-ceruleus acromiodeltoideus (Ito) and Mongolian spot. An ultrastructural study. J Invest Dermatol 1979;73:224-30.
[Google Scholar]
|
| 29. |
Leung AK, Kao CP, Lee TK. Mongolian spots with involvement of the temporal area. Int J Dermatol 2001;40:288-9.
[Google Scholar]
|
| 30. |
Tanyasiri K, Kono T, Groff WF, Higashimori T, Petrovska I, Sakurai H, et al. Mongolian spots with involvement of mandibular area. J Dermatol 2007;34:381-4.
[Google Scholar]
|
| 31. |
Hidano A. Persistent Mongolian spot in the adult. Arch Dermatol 1971;103:680-1.
[Google Scholar]
|
| 32. |
Kikuchi I, Inoue S. Natural history of the Mongolian spot. J Dermatol 1980;7:449-50.
[Google Scholar]
|
| 33. |
Ahn JS, Kim SD, Hwang JH, Youn SW, Kim KH, Park KC. Halo-like disappearance of Mongolian spot combined with café au lait spot. Pediatr Dermatol 1998;15:70-1.
[Google Scholar]
|
| 34. |
Ochiai T, Suzuki Y, Kato T, Shichino H, Chin M, Mugishima H, et al. Natural history of extensive Mongolian spots in mucopolysaccharidosis type II (Hunter syndrome): A survey among 52 Japanese patients. J Eur Acad Dermatol Venereol 2007;21:1082-5.
[Google Scholar]
|
| 35. |
Silengo M, Battistoni G, Spada M. Is there a relationship between extensive Mongolian spots and inborn errors of metabolism? Am J Med Genet 1999;87:276-7.
[Google Scholar]
|
| 36. |
Dweikat I, Libdeh BA, Murrar H, Khalil S, Maraqa N. Gm1 gangliosidosis associated with neonatal-onset of diffuse ecchymoses and Mongolian spots. Indian J Dermatol 2011;56:98-100.
[Google Scholar]
|
| 37. |
Grant BP, Beard JS, de Castro F, Guiglia MC, Hall BD. Extensive Mongolian spots in an infant with Hurler syndrome. Arch Dermatol 1998;134:108-9.
[Google Scholar]
|
| 38. |
Kulkarni KP, Murthy S, Panigrahi I. Child with Mongolian spots and dysostosis multiplex. Indian J Hum Genet 2009;15:38-9.
[Google Scholar]
|
| 39. |
Spranger J. Mucopolysaccharidoses. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JM, Behrman RE, editors. Nelson's textbook of paediatrics. Vol.1., 19 th ed. Philadelphia: Elsevier Saunders; 2011. p. 509-16.
th ed. Philadelphia: Elsevier Saunders; 2011. p. 509-16.'>[Google Scholar]
|
| 40. |
Igawa HH, Ohura T, Suqihara T, Ishikawa T, Kumakiri M. Cleft lip Mongolian spot: Mongolian spot associated with cleft lip. J Am Acad Dermatol 1994;30:566-9.
[Google Scholar]
|
| 41. |
Larralde M, Santos-Muñoz A, Rodríguez Cáceres M, Ciardiullo A. Phacomatosis pigmentovascularis type Va in a 3-month old. Pediatr Dermatol 2008;25:198-200.
[Google Scholar]
|
| 42. |
Hall BD, Cadle RG, Morrill-Cornelius SM, Bay CA. Phakomatosis pigmentovascularis: Implications for severity with special reference to Mongolian Spots associated with Sturge-Weber and Klippel-Trenaunay syndromes. Am J Med Genet A 2007;143A:3047-53.
[Google Scholar]
|
| 43. |
Acebo E, Gardeazábal J, González-Hermosa R, Pérez-Barrio S, Díaz-Pérez JL. Congenital hemangioma: A report of evolution from rapidly involuting to noninvoluting congenital hemangioma with aberrant Mongolian spots. Pediatr Dermatol 2009;26:225-6.
[Google Scholar]
|
| 44. |
Torrelo A, Zambrano A, Happle R. Cutis marmorata telangiectatica congenita and extensive Mongolian spots: Type 5 phacomatosis pigmentovascularis. Br J Dermatol 2003;148:342-5.
[Google Scholar]
|
| 45. |
Wolf R, Wolf D, Davidovici B. Phacomatosis pigmentopigmentalis: Aberrant Mongolian spots and segmental cafe´ au lait macules. Pediatr Dermatol 2009;26:228-9.
[Google Scholar]
|
| 46. |
AlJasser M, Al-Khenaizan S. Cutaneous mimickers of child abuse: A primer for pediatricians. Eur J Pediatr 2008;167:1221-30.
[Google Scholar]
|
| 47. |
Inamdar AC, Palit A. Persistent, aberrant Mongolian spots in Sjögren-Larsson syndrome. Pediatr Dermatol 2007;24:98-9.
[Google Scholar]
|
| 48. |
Kawara S, Takata M, Hirone T, Tomita K, Hamaoka H. A new variety of neurocutaneousmelanosis: Benign leptomeningeal melanocytoma associated with extensive Mongolian spot on the back. Nihon Hifuka Gakkai Zasshi 1989;99:561-6.
[Google Scholar]
|
| 49. |
McLaughlin MR, O'Connor NR, Ham P. Newborn skin: Part II. Birthmarks. Am Fam Physician 2008;77:56-60.
[Google Scholar]
|
| 50. |
Tanyasiri K, Kono T, Groff WF, Higashimori T, Petrovska I, Sakurai H, et al. Mongolian spots with involvement of mandibular area. J Dermatol 2007;34:381-4.
[Google Scholar]
|
| 51. |
Kagami S, Asahina A, Watanabe R, Mimura Y, Shirai A, Hattori N, et al. Laser treatment of 26 Japanese patients with Mongolian spots. Dermatol Surg 2008;34:1689-94.
[Google Scholar]
|
Fulltext Views
92,932
PDF downloads
7,104





