Translate this page into:
Mucocutaneous manifestations of COVID-19-associated mucormycosis: A retrospective cross-sectional study
Corresponding author: Dr. Swastika Suvirya, Department of Dermatology, Venereology and Leprosy, King George’s Medical University, Lucknow, Uttar Pradesh, India. swastika.p@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sachan S, Suvirya S, Yadav K, Gupta P, Saraswat A, Verma P, et al. Mucocutaneous manifestations of COVID-19-associated mucormycosis: A retrospective cross-sectional study. Indian J Dermatol Venereol Leprol 2023;89:510–23.
Abstract
Background
Cutaneous mucormycosis has shown a significant upsurge during the COVID-19 pandemic. Due to the rapid progression and high mortality of cutaneous mucormycosis in this context, it is important to identify it early. However, very few studies report detailed clinical descriptions of cutaneous mucormycosis in COVID-19 patients.
Objectives
To describe mucocutaneous lesions of COVID-19-associated mucormycosis based on clinical morphology and attempt to correlate them with radiological changes.
Methods
A retrospective cross-sectional study was conducted at a tertiary care centre from 1st April to 31st July 2021. Eligibility criteria included hospitalised adult patients of COVID-19-associated mucormycosis with mucocutaneous lesions.
Results
All subjects were recently recovering COVID-19 patients diagnosed with cutaneous mucormycosis. One of fifty-three (2%) patients had primary cutaneous mucormycosis, and all of the rest had secondary cutaneous mucormycosis. Secondary cutaneous mucormycosis lesions presented as cutaneous-abscess in 25/52 (48%), nodulo-pustular lesions in 1/52 (2%), necrotic eschar in 1/52 (2%) and ulcero-necrotic in 1/52 (2%). Mucosal lesions were of three broad sub-types: ulcero-necrotic in 1/52 (2%), pustular in 2/52 (4%) and plaques in 1/52 (2%). Twenty out of fifty-two patients (38%) presented with simultaneous mucosal and cutaneous lesions belonging to the above categories. Magnetic resonance imaging of the face showed variable features of cutaneous and subcutaneous tissue involvement, viz. peripherally enhancing collection in the abscess group, “dot in circle sign” and heterogeneous contrast enhancement in the nodulo-pustular group; and fat stranding with infiltration of subcutaneous tissue in cases with necrotic eschar and ulcero-necrotic lesions.
Limitations
The morphological variety of cutaneous mucormycosis patients in a single-centre study like ours might not be very precise. Thus, there is a need to conduct multi-centric prospective studies with larger sample sizes in the future to substantiate our morphological and radiological findings.
Conclusions
COVID-19-associated mucormycosis patients in our study presented with a few specific types of mucocutaneous manifestations, with distinct magnetic resonance imaging findings. If corroborated by larger studies, these observations would be helpful in the early diagnosis of this serious illness.
Keywords
COVID-19-associated mucormycosis
cutaneous mucormycosis
Plain Language Summary
Black fungus, also termed as mucormycosis, has created a havoc during second wave of coronavirus 2019 disease pandemic in India. The main reason regarding this surge was increased blood sugar levels that occurred either because of injudicious use of systemic corticosteroids used in the treatment of complicated COVID-19 disease or pre-existing uncontrolled adult-onset diabetes mellitus found commonly among Indians. It presented with various clinical forms; of them, skin involvement emerged as a significant clinical finding. Educating people about the cutaneous warning signs of this dreadful fungal infection has become crucial for early detection and management, which could aid in reducing mortality. Very little data is available describing cutaneous involvement of this devastating fungus and their relevant radiological features, which are important diagnostic parameters at initial stage of the disease. Our study attempts to describe several skin manifestations of mucormycosis including few novel and unusual cases. Correlating these cutaneous lesions with radiology added further advantage of early detection when the laboratory reports were awaited.
Introduction
The surge of mucormycosis cases reported from India during the second wave of coronavirus disease 2019 (COVID-19) pandemic has challenged the country’s healthcare system.1 COVID-19-associated mucormycosis has presented with diverse clinical manifestations, often causing diagnostic difficulties.2 The causative fungus belongs to the phylum Mucoromycota, order Mucorales that includes various genera like Rhizopus, Mucor, Rhizomucor and others.3 After rhino-orbital-cerebral mucormycosis and pulmonary mucormycosis, skin is the third most common organ affected by Mucorales.4
Cutaneous mucormycosis is classified into two types: primary and secondary cutaneous mucormycosis.4 Primary cutaneous mucormycosis occurs due to direct inoculation of fungal spores via traumatic disruption of the cutaneous barrier, mostly in immunocompetent patients.4 On the other hand, secondary cutaneous mucormycosis occurs due to contiguous spread of rhino-orbital-cerebral mucormycosis or distant spread in disseminated forms, mainly in immunocompromised patients.4
The primary objective of our study was to describe mucocutaneous manifestations of COVID-19-associated mucormycosis. These lesions were then correlated with radiological findings. The secondary objective was to present a few interesting clinical and radiological features in our patient cohort.
Various morphological forms of primary cutaneous mucormycosis described in the literature include nodules, ulcers, purpuric lesions and swollen plaques.4,5 However, the dermatologic spectrum of secondary cutaneous mucormycosis has not been elucidated.4,5 Clinical heterogeneity of COVID-19-associated mucormycosis needs more exploration with the emergence of atypical mucocutaneous lesions of mucormycosis during the ongoing pandemic. With this aim, we describe the various morphological patterns of cutaneous mucormycosis observed in our hospital during the time of pandemic. Morphological typing and dermato-radiological correlation might help in the early diagnosis and timely management of this dreaded disease.
Materials and Methods
This retrospective cross-sectional study was conducted at King George’s Medical University, Lucknow, Uttar Pradesh, India, a government tertiary care teaching hospital. All COVID-19 patients presenting with definite or probable COVID-19-associated mucormycosis between 1st April 2021 to 31 July 2021 were evaluated. The case definition for active COVID-19 infection was a patient with positive antigen test (reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus-2 infection), while recently recovered COVID-19 patients were those with a history of severe acute respiratory syndrome coronavirus-2 infection within three months from acquiring COVID-19-associated mucormycosis at the time of admission.6 The criteria for defining definite cases of primary cutaneous mucormycosis were based on histopathological evidence of Mucorales.7,8 In contrast, definite or probable secondary cutaneous mucormycosis cases were defined based on the diagnostic criteria of rhino-orbital-cerebral mucormycosis.9 To be classified as definite secondary cutaneous mucormycosis, patients had to manifest typical clinical features (facial or eyelid swelling/necrosis/pain), radiological signs of tissue infiltration, and confirmatory laboratory reports (histopathological/potassium hydroxide/fungal culture), while patients with only positive clinical and radiological findings without laboratory confirmation were classified as probable secondary cutaneous mucormycosis.
A waiver from informed consent was requested from the ethical committee. As the study was retrospective in nature, the request was accepted (110thECM II IIA/P15). Patients’ records were retrieved electronically from the database by searching digital medical records and manually from the case record sheets. Out of 300 COVID-19 related mucormycosis patients, 71 had cutaneous lesions. Of these patients with skin lesions, 11 were excluded because of the non-availability of consent for photographs. Seven more patients were further not analysed due to incomplete clinical and laboratory information. Fifty-three patients with complete medical records were taken up for analysis. Complete medical records consisted of case sheets, clinical images, all laboratory investigation results, radiological images and reports of both non-contrast and contrast-enhanced pre-operative magnetic resonance imaging. As per the protocol practised at our centre, non-invasive radiological investigations were performed first, followed by nasal endoscopy and biopsy or surgical debridement for diagnostic (microbiological and histopathological evaluation) and therapeutic purposes in cases of suspected mucormycosis.
Clinical and radiological images of resolving cutaneous lesions were available in five cases.
Radiology
Reports and images of pre-operative magnetic resonance imaging, non-contrast and contrast-enhanced, of the face, paranasal sinuses, orbit and cranium of the recruited patients, were retrieved from the electronic database. In addition, reports and images of pre and post-operative computed tomography/post-operative magnetic resonance imaging were also collected depending on the availability. Five such images were available for review.
Description of morphological and radiological patterns of COVID-19-associated mucormycosis and their correlation
Morphological description of COVID-19-associated mucormycosis was done by a panel of four expert dermatologists who analysed clinical images with associated clinical information provided in case sheets.Thus a final decision was made on the cutaneous morphological pattern of COVID-19-associated mucormycosis. In addition, two expert radiologists,did a dermato-radiological correlation in discussion with expert dermatologists of the above panel based on clinical and radiological image analysis. All the four expert dermatologists that are SS, PV, SS, UC and two radiologists AP, SK who are part of this study are authors of the manuscript.
Microbiology
Results of microscopy and fungal culture from pus/nasal/maxillary/cutaneous tissue specimens were obtained. Direct microscopy from biological specimens was done using 10-20% potassium hydroxide solution and enhanced by calcofluor white for easy visibility. The culture medium used for the growth of Mucorales was plain Sabouraud dextrose agar or Sabouraud dextrose agar with chloramphenicol or potato dextrose agar. Species identification was done by conventional methods like lactophenol cotton blue stain and matrix-assisted laser desorption ionisation-time of flight mass spectrometry in all the culture-positive specimens.
Histopathology
Evidence of characteristic features of Mucorales in haematoxylin and eosin and periodic acid-Schiff stained histopathological sections were available for evaluation.
Statistical analysis
The general characteristics and the socio-demographic data of the patients were summarised by mean ± standard deviation and percentages and proportions, whichever was appropriate. For data analysis, IBM SPSS version 22 was used.
Results
There were 23 (43%) females and 30 (57%) males out of a total of 53 patients with cutaneous mucormycosis. The mean age of onset was 50.07 ± 11.64 years (range 21-71 years). The single primary cutaneous mucormycosis patient was a female aged 21 years. The detailed epidemiological features of patients, risk factors of COVID-19-associated mucormycosis and other relevant data are described in Table 1.
| Age | Range 21-71 years Mean 50.07 ± 11.64 years |
| Gender | Male 30/53 (57%) Female 23/53 (43%) |
| COVID-19a status | All were recovered COVID-19 patients < 3 months [RT-PCR negative at time of recruitment] |
| Risk factors for CAMb present in the current analysis | ● Hyperglycemia at admission 53/53 (100%) ● Diabetic ketoacidosis 2/53 (4%) ● History of COVID-19 infection < 3 months 53/53 (100%) ● Systemic steroids for moderate-severe COVID-19 50/53 (94%) ● Unmonitored/unhygienic oxygen therapy at home during active COVID-19 infection due to shortage of hospital beds 9/53 (17%) |
| Hyperglycemia at admission | Mean random blood sugar (RBS) 295.60 ± 48.69 mg/dl Range of RBS 205-411 mg/dl |
| Pre-existing diabetes mellitus (DM) 47/53 (89%) Type of DM ● DM type II (47) ● DM type I (0) New-onset DM/hyperglycemia 6/53 (11%) |
|
| Types of CMc | PCM 1/53 (2%) SCM following ROCM 52/53 (98%) |
| Microbiological evidence (yes/no) if yes, number of patients with positive findings | PCM no SCM yes, KOH (43 patients), fungal culture (39 patients) |
| Histopathological evidence (yes/no) if yes, number of patients with positive findings | PCM yes 1 patient SCM yes, 30 patients |
| ROCMd diagnosis | Definite 43/52 (83%) Probable 9/52 (17%) Possible none |
| Treatment of mucormycosis | Medical Systemic antifungals-liposomal amphotericin B/Posaconazole-53/53 (100%) Surgical debridement 53/53 (100%) |
aCoronavirus disease 2019, bCOVID-19-associated mucormycosis, cCutaneous mucormycosis, dRhino-orbital-cerebral mucormycosis, RT-PCR: Reverse-transcriptase polymerase chain reaction, KOH: Potassium hydroxide, PCM: Primary cutaneous mucormycosis, SCM: Secondary cutaneous mucormycosis, DM: Diabetes mellitus, RBS: Random blood sugar
All patients in our analysis had a recent history (≤3 months) of COVID-19 illness diagnosed by reverse-transcriptase polymerase chain reaction test prior to the development of cutaneous mucormycosis.
Morphological subtypes of cutaneous COVID-19-associated mucormycosis
Out of 53 patients with cutaneous manifestation of COVID-19-associated mucormycosis, one patient had definite primary cutaneous mucormycosis, while the rest 52 (98%) patients had secondary cutaneous mucormycosis following rhino-orbital-cerebral mucormycosis. All patients had lesions localised only to the face. The sole patient of primary cutaneous mucormycosis presented with a blackish scaly plaque on the forehead studded with a few pustules.
Among 52 patients with secondary cutaneous mucormycosis, 43 (83%) patients had definite rhino-orbital-cerebral mucormycosis and 9 (17%) had probable rhino-orbital-cerebral mucormycosis. Various morphological patterns of secondary cutaneous mucormycosis were noted on skin, mucosa or both. The secondary cutaneous mucormycosis lesions observed were classified into various groups according to clinical morphology and site of involvement. These presentations were: cutaneous abscess in 25/52(48%), nodulo-pustular lesions in 1/52 (2%), necrotic eschar 1/52 (2%) and ulcero-necrotic lesions 1/52 (2%).
Mucosal lesions were ulcero-necrotic in 1/52 (2%), pustules in 2/52 (4%) and plaque in 1/52 (2%).
A few patients [20/52 (38%)] had a combination of mucosal and cutaneous lesions with morphological forms as described above in each mucosal or cutaneous category. Out of these 20 patients with mucocutaneous secondary cutaneous mucormycosis, 14 had a combination of cutaneous abscess and mucosal ulcero-necrotic lesions, 5 had a combination of cutaneous nodulo-pustular lesions and mucosal ulcero-necrotic lesions and 1 patient of cutaneous ulcero-necrotic lesion also had mucosal ulcero-necrotic lesion. The clinical pictures of some of these patients are depicted in Figures 1a, 2a, 3a, 4a, 5a, 6a, 7a and 8a.
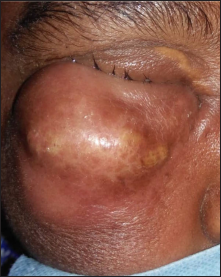
- A large contiguous abscess of size 6 × 8 cm over right cheek and ipsilateral eyelids

- Corresponding axial T2 weighted MR image shows signal intensity alteration, hyperintense area (arrow) with adjacent edema, fat stranding in the premaxillary and infraorbital region with bilateral maxillary sinusitis
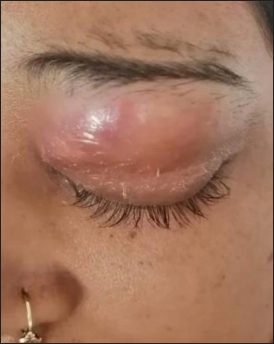
- Left upper eyelid abscess of size 2.5 × 5.5 cm
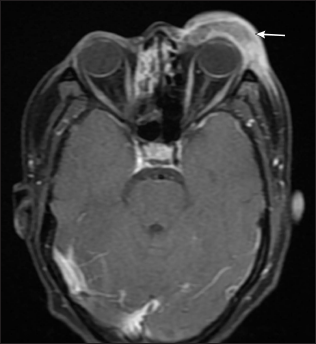
- Corresponding axial post contrast T1 weighted MR image shows a peripherally enhancing collection involving the left upper eyelid (arrow)
![Multiple nodulo-pustular lesions over right cheek [size ranging from 0.5 × 0.5 cm to 1 × 1.5 cm]](/content/126/2023/89/4/img/IJDVL-89-4-510-g005.png)
- Multiple nodulo-pustular lesions over right cheek [size ranging from 0.5 × 0.5 cm to 1 × 1.5 cm]

- Corresponding axial T2 weighted MR image shows a multilobulated mass depicting ‘dot in circle’ sign in right premaxillary region (arrow)

- An ulcer of size 1.5 x 3.5 cm with irregular margin and surrounding necrosis on left temporal region and left ear malignant otitis externa
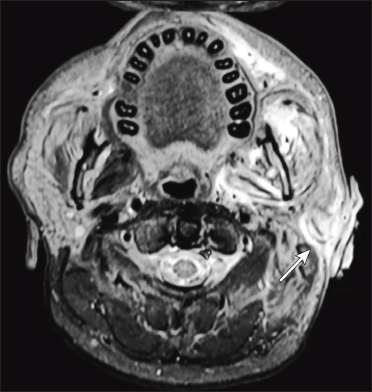
- Corresponding axial T2 fat suppressed MR shows bilateral maxillary sinusitis with altered signal intensity and associated inflammatory changes in left masticator space extending to involve left external auditory meatus and pinna (arrow) leading to its destruction
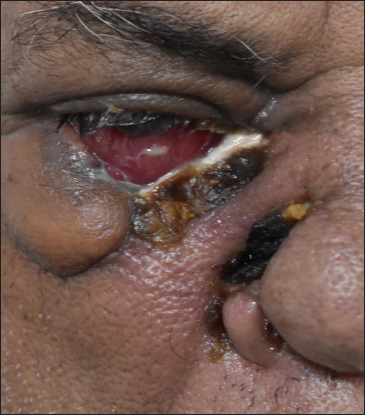
- Muco-cutaneous ulcer of size 2 x 3.5 cm with irregular necrosed rolled out edge masquerading a rodent ulcer at the junction of right palpebral conjunctiva and skin of lower eyelid
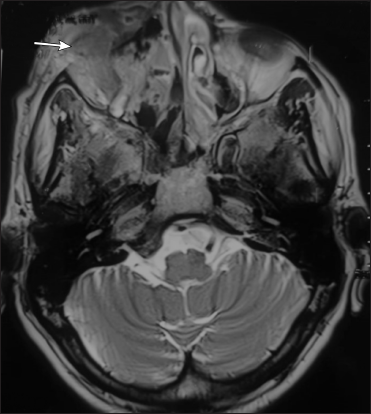
- Corresponding axial T2 fat suppressed MR image shows ill-defined soft tissue mass in right premaxillary region involving overlying skin and subcutaneous tissue with mild skin irregularity in right infra-orbital region (arrow)
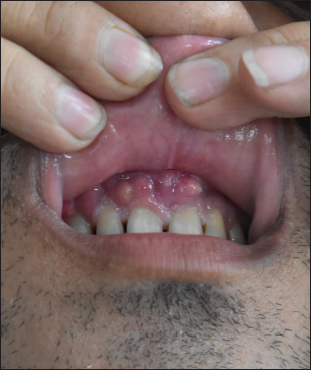
- Multiple gingival pustules on the upper gingiva (anterior aspect)

- Corresponding axial T2 weighted MR image shows soft tissue thickening of superior gingivo-buccal sulcus on right side (arrow)
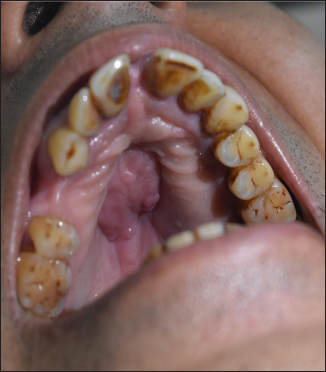
- Mucosal corrugated plaque of size 2 × 3 cm over hard palate
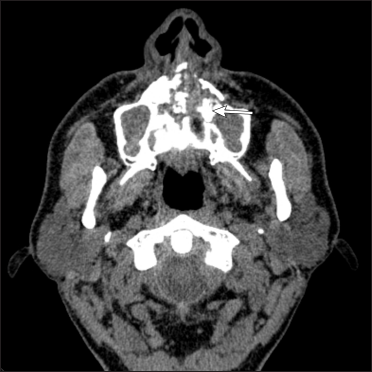
- Corresponding axial non contrast CT scan shows bilateral maxillary sinusitis with moth-eaten erosion of hard palate
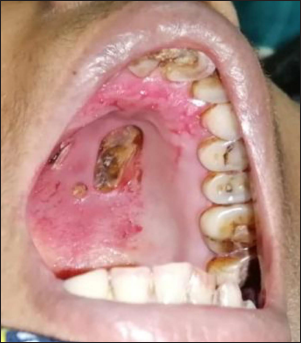
- Multiple ulcero-necrotic lesions of sizes 0.5 × 0.5 cm to 1.5 × 2 cm, exposing underlying bone of hard palate
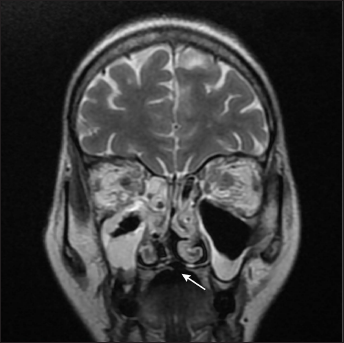
- Corresponding coronal T2 weighted MR image shows focal defect in the hard palate (arrow) with bilateral maxillary, ethmoid sinusitis and orbital cellulitis
Atypical morphological variants of COVID-19-associated mucormycosis
Two out of 52 secondary cutaneous mucormycosis patients had cutaneous ulcero-necrotic lesions on the face. One was a case of probable rhino-orbital-cerebral mucormycosis, having supportive radiological findings. The other was a proven case of secondary cutaneous mucormycosis (rhino-orbital-cerebral mucormycosis), who also had associated malignant otitis externa of the left ear.
In addition, 1/52 (2%) had mucocutaneous ulcero-necrotic lesion at the junction of right palpebral conjunctiva and ipsilateral lower eyelid skin with a rodent ulcer-like appearance.
Cutaneous/subcutaneous abscess over cheeks, eyelids, or both was the commonest morphology observed in a majority of our secondary cutaneous mucormycosis patients with rhino-orbital-cerebral mucormycosis.
Secondary cutaneous mucormycosis patients with cutaneous nodulo-pustular lesions over cheeks clinically mimicked the acute phase of facial actinomycosis.
Dermato-radiological correlation
The contrast-enhanced magnetic resonance imaging (CEMRI) of primary cutaneous mucormycosis lesion showed enhancing lesions in cutaneous and subcutaneous tissue. Cutaneous extension of rhino-orbital-cerebral mucormycosis presenting as secondary cutaneous mucormycosis showed more underlying destruction in radiology than was evident clinically.
CEMRI of cutaneous abscess group of secondary cutaneous mucormycosis variety showed a thick-walled peripherally enhancing collection in subcutaneous tissue.
Magnetic resonance imaging (MRI) of the nodulo-pustular group depicted subcutaneous multilobulated nodular masses that mimicked the ‘dot in circle sign’. In addition heterogeneous contrast enhancement of subcutaneous tissue was also noted in the CEMRI of this group.
In the case of cutaneous and mucocutaneous ulcero-necrotic lesions, soft tissue enhancement, including subcutaneous oedema, was visible on MRI.
The magnetic resonance imaging of the patient with cutaneous necrotic eschar showed diffuse fat stranding in subcutis. In addition, severe bony destruction in the contrast-enhanced computed tomography (CECT) of orbits, nasal turbinates, sinus walls and cranium were also evident in this patient.
Mucosal ulcero-necrotic lesions showed signal intensity alteration of the hard palate in CEMRI. Mucosal pustular lesions revealed soft tissue thickening in the upper gingivo-buccal sulcus and altered signal intensity of the hard palate in CEMRI.
On the other hand, a mucosal plaque appeared as a heterogeneously enhancing exophytic soft tissue lesion and evident erosion of the hard palate and sinus involvement on CEMRI.
CECT of all mucosal lesions showed mucosal thickening of the hard palate with associated cortical erosion.
Other interesting radiological findings apart from sino-nasal involvement, which we observed were common in all the morphological groups of secondary cutaneous mucormycosis. A few such findings were orbital cellulitis, optic neuritis and panophthalmitis (n = 17), cavernous sinus infiltration/thrombosis (n = 15), cerebral abscess-frontal lobe/temporal lobe (n = 3), erosion of cribriform plates (n = 2), meningitis (n = 2), cerebral artery stenosis (n = 13) and common carotid artery stenosis (n = 1). Radiological images of patients are depicted in Figures 1b, 2b, 3b, 4b, 5b, 6b, 7b and 8b along with corresponding clinical images in Figures 1a, 2a, 3a, 4a, 5a, 6a, 7a and 8a. Clinico-radiological characteristics of lesions and rare, interesting radiological features are discussed in Table 2.
| Morphological subtypes and anatomical distribution (n = 53) | Dermato-radiological co-relating features of face and interesting findings if any | Supplementary radiological findings of orbit, brain and PNSe irrespective of morphological type |
|---|---|---|
| A. PCMa (n = 1) |
MRIc-skin and subcutaneous infiltration, no underlying bony erosion of right fronto-temporal region with mild post contrast enhancement. Morphology- Beatty et al.,11 Wang et al.12 Radiology- Putthirangsiwong B et al.13 & Aljehani M et al.14 |
|
| B. SCMb (n = 52) | ||
| I) CUTANEOUS LESIONS | ||
| a) Abscess (n = 25) |
MRI-peripherally enhancing collection at premaxillary area and/or eyelids Morphology - Rare presentation-El-Kholy et al.19 Radiology -Therakathu et al.15 |
Orbital cellulitis,optic neuritis, panophthalmitis [MRI] n = 18 |
| b) Nodulo-pustular (facial actinomycosis like) (n = 1) |
MRI- Multilobulated mass with heterogenous enhancement at premaxillary area Periorbital soft tissue enhancement in case of eyelid involvement |
Cavernous sinus infiltration/thrombosis [MRI] n = 16 |
| Clinical mimic- Acute form of Facial actinomycosis | Common carotid artery stenosis [MRI] n = 1 | |
|
Radiological mimic- Dot in circle like mycetoma, MRI-Laohawiriyakamol et al.25 Radiology-(Fungal etiology)— Sanghvi et al.24 |
||
| c) Ulcer-necrotic (n = 1) |
Cutaneous ulcero-necrotic lesion- associated with malignant otitis externa. (n = 1) MRI-skin ulceration with underlying ill-defined heterogenously enhancing soft tissue. |
Cerebral abscess [MRI] -frontal lobe (n = 1), temporal lobe (n = 2) |
| d) Necrotic eschar (n = 1) | MRI- Ill-defined heterogeneously enhancing soft tissue of premaxillary areas bilaterally +associated diffuse skin and subcutaneous tissue thickening +fat stranding | Erosion of cribriform plates [MRI] n = 3 |
| II) MUCOSAL LESIONS | ||
| a) Ulcero-necrotic (n = 1) | MRI-Signal intensity alteration noted over hard palate | |
| b) Mucosal pustules (n = 2) |
MRI-Soft tissue thickening in upper gingiva-buccal sulcus CECTd-Moth eaten appearance of hard palate. (n = 1) |
|
| c) Mucosal plaque (n = 1) | MRI- Ill-defined heterogeneously enhancing exophytic soft tissue noted involving hard palate with erosion of the hard palate and mucosal edema. | |
|
CECT-Moth eaten appearance of hard palate. Mucosal pustules & mucosal plaque Radiology-Mucormycotic moth eaten appearance of maxilla is considered rare Pandilwar et al.29 Mucosal Plaque Morphology-Palatal swelling is infrequent-Mohanty et al.28 |
||
| III) Mucocutaneous (n = 20) | ||
| a) Cutaneous Ulcero-necrotic + Mucosal Ulcer-necrotic (n = 1) | ||
| b) Cutaneous Abscess + Mucosal Ulcer-necrotic (n = 14) | Clinical mimic of Cutaneous Ulcero-necrotic +Mucosal Ulcer-necrotic: rodent ulcer like | |
| c) Cutaneous Nodulo-pustular + Mucosal Ulcer-necrotic (n = 5) | ||
aPrimary cutaneous mucormycosis, bSecondary cutaneous mucormycosis, cMagnetic resonance imaging, dContrast-enhanced computed tomography, eParanasal sinuses
Mycology
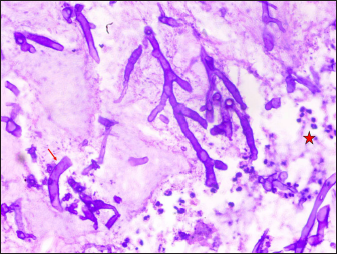
Refractile broad aseptate fungal hyphae irregularly branched at 90o angle with characteristic umbrella-shaped sporangium were noticed in potassium hydroxide mount of 43 of 52 (83%) secondary cutaneous mucormycosis patients. In addition, 39 of 52 (75%) patients with secondary cutaneous mucormycosis showed cotton candy colonies in fungal culture media [Figures 9a and b]. Two species were identified Rhizopus arrhizus in 29 of 52 (56%) patients and Rhizopus microsporus in 10 of 52 (19%) patients with the help of lactophenol cotton blue mount and matrix-assisted laser desorption ionisation-time of flight mass spectrometry [Figure 9c]. Histopathological evaluation with periodic acid-Schiff and haematoxylin and eosin stain showed broad aseptate hyphae folded in a ribbon-like manner and branched at 90 angle to each other in one primary cutaneous mucormycosis patient and 30 of 52 (58%) secondary cutaneous mucormycosis patients [Figures 10 a-d].
![KOH MOUNT - Broad aseptate hyphae with irregular branching (red arrow) and umbrella shaped sporangium (black arrow) [45×]](/content/126/2023/89/4/img/IJDVL-89-4-510-g017.png)
- KOH MOUNT - Broad aseptate hyphae with irregular branching (red arrow) and umbrella shaped sporangium (black arrow) [45×]

- Cotton candy colony in Sabouraud dextrose agar culture medium
![LCB Mount of Rhizopus arrhizus- shows circular sporangium (black arrow) with rhizoids (white arrow) [45×]](/content/126/2023/89/4/img/IJDVL-89-4-510-g019.png)
- LCB Mount of Rhizopus arrhizus- shows circular sporangium (black arrow) with rhizoids (white arrow) [45×]
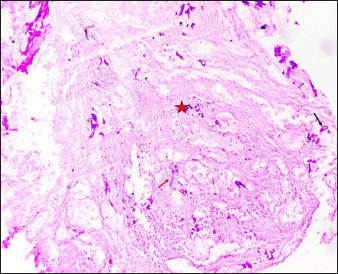
- Case of PCM showing abundant necrosis (asterisk) and few scattered hyphae (arrows). (Periodic Acid Schiff, ×50)
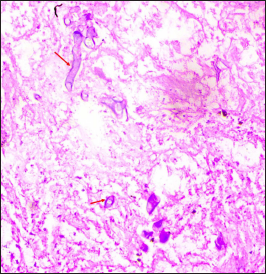
- Case of PCM showing broad based aseptate hyphae (arrows) on a background of abundant necrosis. (Periodic Acid Schiff, ×200)
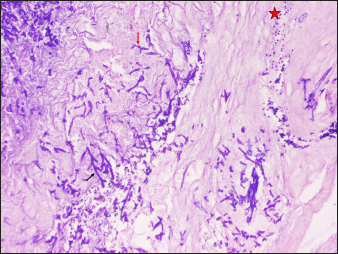
- Case of SCM showing numerous hyphae (arrows) with neutrophilic infiltrate in the background (Periodic Acid Schiff, ×100)
- Case of SCM showing numerous broad based hyphae (arrow) with neutrophilic infiltrate in the background (Periodic Acid Schiff, ×200)
Additional analysis
Depending on the anatomical sites affected, secondary cutaneous mucormycosis patients were managed with systemic antifungals and surgical debridement. Additionally, in the patient with malignant otitis externa, a combination of piperacillin plus tazobactam and clindamycin was given in accordance with the culture sensitivity report. The clinical and radiological resolution pattern during the period of hospital stay, according to available images of a few selected patients in the cutaneous abscess group (n = 1), cutaneous nodulo-pustular group (n = 1) and mucosal pustules group (n = 1) are illustrated in Figures 11 a-d. Ten of fifty-two (19%) secondary cutaneous mucormycosis patients died during their hospital stay.
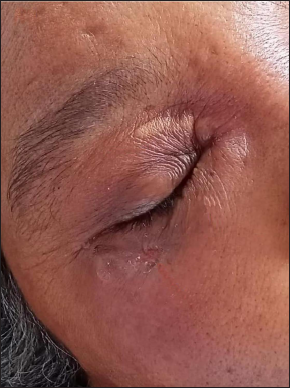
- Resolving abscess with wrinkled surface over right eyelids and an erosion below
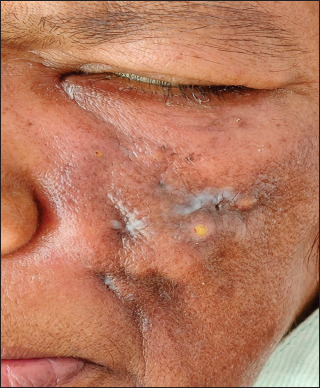
- Nodulo-pustular lesion healing with puckered scarring and surrounding residual erythema on left cheek
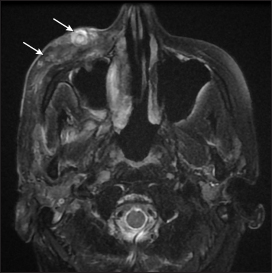
- Axial T2 weighted fat suppressed image after debridement shows resolving nodules with hypointense centre in skin and subcutaneous tissue in a patient of cutaneous nodulo-pustular group (arrows)
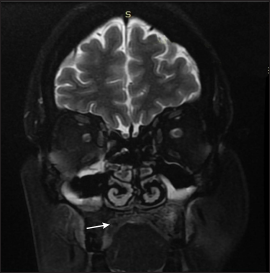
- Coronal T2 weighted fat suppressed MR image after debridement shows hypointense soft tissue thickening of the hard palate (arrow) of mucosal-pustule group
Discussion
The outbreak of mucormycosis cases during the current severe acute respiratory syndrome coronavirus-2 pandemic is most likely related to either pre-existing or new-onset hyperglycaemia, as evident in all our patients, making uncontrolled blood sugar an independent risk factor.1 Demographic profile, data related to risk factors of COVID-19-associated mucormycosis, category of cutaneous mucormycosis and laboratory records of our patients as described in Table 1 are similar to risk factors described in contemporary studies.1,4,6
Very few cases of primary cutaneous mucormycosis have been reported worldwide in covid-associated mucormycosis.10 Similarly, in the current retrospective study, we could find only one patient with primary cutaneous mucormycosis. The morphological pattern found was a scaly blackish plaque on the right side of the forehead with few superficial pustules over the surface, which is an infrequently described clinical presentation for the same.11,12 For confirmation of primary cutaneous mucormycosis, histopathology has a higher yield than microbiology, corroborating with our case.5 CEMRI findings like mild enhancing cutaneous and subcutaneous tissue were similar to changes described in few case reports.13,14 Early initiation of amphotericin B, strict blood sugar control and surgical intervention may have halted our patient’s disease progression to a severe necrotic stage.
Face and orbit are considered common sites for the extra-sinus extension of rhino-orbital-cerebral mucormycosis; the same was observed in our secondary cutaneous mucormycosis patients.15 The role of radiology is crucial in rhino-orbital-cerebral mucormycosis as underlying tissue infiltration in the initial phase, especially deep soft tissue affection of the face, could indicate an early sign of disease even before the Mucorales are visible on microscopy, as was evident in the majority of our cases.16
There is a scarcity of literature describing the morphology of secondary cutaneous mucormycosis. Cutaneous necrosis and eschar formation are considered the most frequent presentations because of the angio-invasive property of Mucorales.4,5 Contrary to this, we found only one patient with typical cutaneous necrotic eschar involving the entire right half of the face, nose and medial portion of the left cheek. Fat stranding and ill-defined heterogeneously enhancing skin and subcutaneous tissue in the bilateral premaxillary region in combination with pansinusitis observed in CEMRI of this patient indicated soft tissue necrosis occurring possibly due to angio-invasive mucormycosis.15,16 Histopathological evidence of characteristic hyphae of Mucorales confirmed the diagnosis in this patient.
Among 52 secondary cutaneous mucormycosis patients, only two had an ulcero-necrotic lesion on the face.4,5 Out of these, one patient had associated malignant otitis externa of the left ear, a rare presentation. Pseudomonas aeruginosa was detected in pus collected from the affected external auditory canal in this case.17 However, tissue biopsied from the same site also showed characteristic hyphae of Mucorales. Fungal aetiology was further supported by magnetic resonance imaging that showed bilateral maxillary sinusitis and an ill-defined soft tissue mass probably fungal in nature encroaching the left middle ear and left external auditory canal.15 Even though, we could not establish whether it was fungal malignant otitis externa developing over improperly treated bacterial malignant otitis externa or vice versa. Thus, we considered mixed infection to be the cause of malignant otitis externa in this patient since both Pseudomonas and Mucorales individually are known to cause malignant otitis externa but not in amalgamation, making it a unique presentation during the current pandemic with no such case reported to date.14,17
In another patient with definite rhino-orbital-cerebral mucormycosis with secondary cutaneous mucormycosis presenting with a ulcero-necrotic lesion, the disease presented with features similar to a nodulo-ulcerative-type basal cell carcinoma. However, the underlying sinus and orbital tissue involvement in CEMRI suggested rhino-orbital-cerebral mucormycosis with cutaneous extension, which guided the early initiation of antifungal therapy. Later, the evidence of Mucorales was also confirmed by visualisation of the causative fungus in both microbiological and histopathological examinations. This case emphasises the importance of clinico-radiological correlation in early diagnosis and treatment since microbiological or biopsy reports usually take a longer time.
Suppuration in cutaneous mucormycosis has been described earlier, but clinically frank abscess has hitherto been considered a rare manifestation, which was contrary to our observation.12,18,19 The possibility of a secondary bacterial infection or combined infection with actinomycosis, reported in the literature as a cause for this suppuration was ruled out in all patients in the cutaneous abscess group by negative microbiological results from pus specimens for both these organisms.20,21 Mucorales as the cause of abscess were further supported by the presence of characteristic fungal hyphae in pus on potassium hydroxide mounts (n = 8) and positive growth on fungal culture (n = 4). Interestingly, two of our patients also had cerebral abscesses, an uncommon finding reported previously.22,23 Pus from cerebral abscess obtained by craniectomy depicted characteristic hyphae of Mucorales on potassium hydroxide mount. CEMRI of the face in the abscess group also revealed thick-walled peripherally enhancing fluid collection in subcutaneous tissue, as mentioned in a few recent studies.15,24
Secondary cutaneous mucormycosis patients of the cutaneous nodulo-pustular variety whose lesions clinically mimicked the acute phase of facial actinomycosis were also unusual. T2 weighted magnetic resonance imaging finding of subcutaneous multilobulated nodular masses that mimicked the ‘dot in circle sign’ of mycetoma in these patients could be explained as hyperintense peripheral soft tissue infiltration depicting a circle and hypointense necrosis in the centre manifesting as a dot. To date, this novel clinical and radiological observation has not been reported in mucormycosis.25 In addition, heterogenous contrast-enhancement was noticed in CEMRI of a nodulo-pustular lesion, which according to literature is suggestive of a fungal aetiology.15,24
Mucosal ulcero-necrotic lesion over the hard palate is a notable finding of secondary cutaneous mucormycosis. Similar findings were observed in our patients either alone or in combination with cutaneous lesions.26 Among them, one patient had a palatal ulcero-necrotic lesion and sinus involvement without cutaneous extension, whereas 20 patients had the same mucosal morphology along with cutaneous lesions (abscess/nodulo-pustular/ulcero-necrotic) and sino-orbital involvement. Nonetheless, unlike palatal ulcers and necrosis, gingival pustules and palatal swellings observed here are lesser-known descriptions of secondary cutaneous mucormycosis.27,28 Mucosal pustules over the upper gingiva were observed in two of our patients. In one patient with mucosal pustules, CECT of the hard palate revealed osteomyelitis with a moth-eaten appearance, which is again a rarely described entity.30 Mucosal plaque over hard palate had corrugated surface noticed in a patient of probable rhino-orbital-cerebral mucormycosis; very few such presentations have been mentioned in literature.28,30 The corresponding CEMRI of palatal plaque showed an ill-defined heterogeneously enhancing exophytic soft tissue lesion with the erosion of the hard palate suggestive of fungal infection.24
Overall, Rhizopus arrhizus is the most common Mucorales causing various forms of COVID-19-associated mucormycosis, including cutaneous disease and rhino-orbital-cerebral mucormycosis.4,5 Likewise, in our analysis, Rhizopus arrhizus was frequently isolated, followed by Rhizopus microsporus4,5
Lastly, whether the unique morphological presentations of COVID-19-associated mucormycosis noted in this study can be attributed to severe acute respiratory syndrome coronavirus-2 induced altered virulence of Mucorales or immunity of the host or both needs to be evaluated in future studies.
Limitations
The spectral distribution of cutaneous mucormycosis patients in a single-centre study like ours might not be very precise. Therefore, multi-centric prospective studies with larger sample sizes are required in the future to substantiate our morphological and radiological findings.
Conclusion
This study highlights several novel and atypical presentations of cutaneous mucormycosis associated with COVID-19. It may help characterise its mucocutaneous patterns further and hold relevance in identifying early infections. Though in cases of rhino-orbital-cerebral mucormycosis, the radiological features of underlying structures, namely sinus, orbit and brain, are essential for planning surgical treatment or defining prognosis. Dermato-radiological correlation may help reduce morbidity and mortality if dermatologists suspect cutaneous mucormycosis early, by close clinical observation and supportive radiologic findings. It may also help initiate prompt multi-modality management of this devastating fungal infection.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15:102146.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes, COVID 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab Syndr. 2021;15:102196.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-associated mucormycosis: Evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl Trop Dis. 2021;15:e0009921.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis with cutaneous involvement. A retrospective study of 115 cases at a tertiary care hospital in Mexico. Australas J Dermatol. 2021;62:162-7.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-associated mucormycosis presenting to the Emergency Department-An observational study of 70 patients. QJM. 2021;114:464-70.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and diagnosis of mucormycosis: An update. J Fungi (Basel). 2020;6:265.
- [CrossRef] [PubMed] [Google Scholar]
- A guide to investigating suspected outbreaks of mucormycosis in healthcare. J Fungi (Basel). 2019;5:69.
- [CrossRef] [PubMed] [Google Scholar]
- Code mucor: Guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361-5.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient-Case report and review of literature. J Mycol Med. 2021;31:101125.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Primary cutaneous mucormycosis developing after incision and drainage of a subcutaneous abscess in an immunocompetent host. BMJ Case Rep. 2016;2016:bcr2015213700.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous mucormycosis caused by Rhizopus microsporus in an immunocompetent patient: A case report and review of literature. Medicine (Baltimore). 2018;97:e11141.
- [CrossRef] [PubMed] [Google Scholar]
- Periocular cutaneous mucormycosis caused by Saksenaea erythrospora. J Pediatr Infect Dis. 2019;14:209-12.
- [Google Scholar]
- Corrigendum to “A Case Report of Complete Resolution of Auricular Mucormycosis in an 18-Month-Old Diabetic Child”. Case Rep Otolaryngol. 2021;2021:9794624.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Imaging features of rhinocerebral mucormycosis: A study of 43 patients. Egypt J Radiol Nucl Med. 2018;49:447-52.
- [CrossRef] [Google Scholar]
- Imaging features of rhinocerebral mucormycosis: From onset to vascular complications. Acta Radiol. 2022;63:232-44.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and microbiological profile of malignant otitis externa in a tertiary care center. J Evol Med Dent Sci. 2020;45:3356-61.
- [Google Scholar]
- Rhino-orbito cerebral mucormycosis - uncontrolled, untimely and unsaved. Saudi J Med Med Sci. 2019;04:759-61.
- [CrossRef] [Google Scholar]
- Invasive fungal sinusitis in post COVID-19 patients: A new clinical entity. Laryngoscope. 2021;131:2652-8.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 associated mucormycosis: Staging and management recommendations (Report of a multi-disciplinary expert committee) J Oral Biol Craniofac Res. 2021;11:569-80.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinocerebral mucormycosis associated with actinomycosis in a diabetic patient: A rare presentation. J Oral Maxillofac Pathol. 2019;23:122-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mucormycosis of the central nervous system. J Fungi (Basel). 2019;5:59.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis creeping along the nerves in an immunocompetent individual. J Radiol Case Rep. 2019;13:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of COVID-19-associated craniofacial mucormycosis: A black and white review of the “black fungus”. Clin Radiol. 2021;76:812-9.
- [CrossRef] [PubMed] [Google Scholar]
- The “dot-in-circle” sign in musculoskeletal mycetoma on magnetic resonance imaging and ultrasonography. Springerplus. 2014;3:671.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oral mucormycosis: A new threat to COVID patients? A guide to oral health. J Dental Health Oral Res. 2021;2:1-7.
- [Google Scholar]
- Mucormycotic osteomyelitis involving the maxilla: A rare case report and review of the literature. Case Rep Infect Dis. 2019;2019:8459296.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinomaxillary mucormycosis masquerading as chronic osteomyelitis: A series of four rare cases with review of literature. J Indian Acad Oral Med Radiol. 2012;24:315-23.
- [CrossRef] [Google Scholar]
- Mucormycosis: A rare entity with rising clinical presentation in immunocompromised hosts. Int J Surg Case Rep. 2020;77:57-61.
- [CrossRef] [PubMed] [Google Scholar]
- Rhino-maxillary mucormycosis in an immunocompetent individual: Importance of early diagnosis. J Indian Acad Oral Med Radiol. 2014;26:315-8.
- [CrossRef] [Google Scholar]
- Malignant otitis externa: A retrospective analysis and treatment outcomes. Turk Arch Otorhinolaryngol. 2018;56:106-10.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis: A devastating fungal infection in diabetics. J Coll Physicians Surg Pak. 2005;15:43-5.
- [PubMed] [Google Scholar]






