Translate this page into:
Multicentric squamous cell carcinoma over lesions of porokeratosis palmaris et plantaris disseminata and giant porokeratosis
Correspondence Address:
Sujata Sengupta
Department of Dermatology, R K M Seva Pratisthan and Vivekananda Institute of Medical Sciences, Kolkata
India
| How to cite this article: Sengupta S, Das JK, Gangopadhyay A. Multicentric squamous cell carcinoma over lesions of porokeratosis palmaris et plantaris disseminata and giant porokeratosis. Indian J Dermatol Venereol Leprol 2005;71:414-416 |
Abstract
Porokeratosis is a specific disorder of keratinization that has five clinical types and shows a characteristic 'cornoid lamella' on histopathology. Malignant degeneration has been described in all forms of porokeratosis. To the best of our knowledge, this is the first Indian report of multicentric squamous cell carcinoma complicating porokeratosis.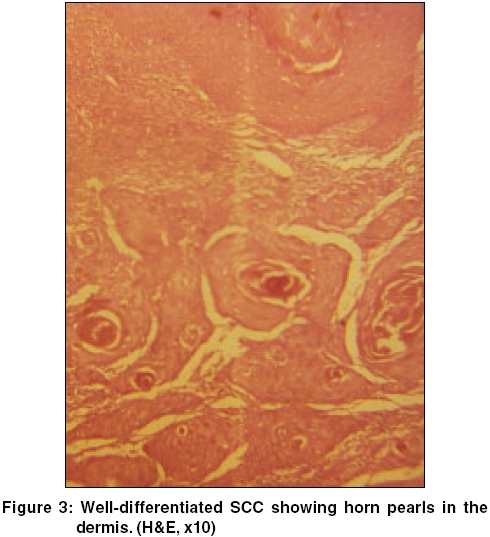 |
 |
 |
 |
 |
 |
Introduction
Porokeratosis is a clonal disorder of keratinization characterized by one or more atrophic patches surrounded by a clinically and histologically distinct ridge-like border called ′cornoid lamella′. Fine major clinical variants are recognized - classical porokeratosis of Mibelli (PM), disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis (LP), porokeratosis palmaris et plantaris disseminata (PPPD), and punctate porokeratosis (PP).[1] Besides these, rare variants like giant porokeratosis and hyperkeratotic and verrucous types have been described. Malignant degeneration has been described in all forms of porokeratosis but LP has the highest incidence of malignant transformation into squamous, bowenoid or basal cell carcinoma.[2] A coexistence of PPPD with giant porokeratosis, complicated by multicentric squamous cell carcinoma (SCC) is hitherto unreported in Indian patients.
Case report
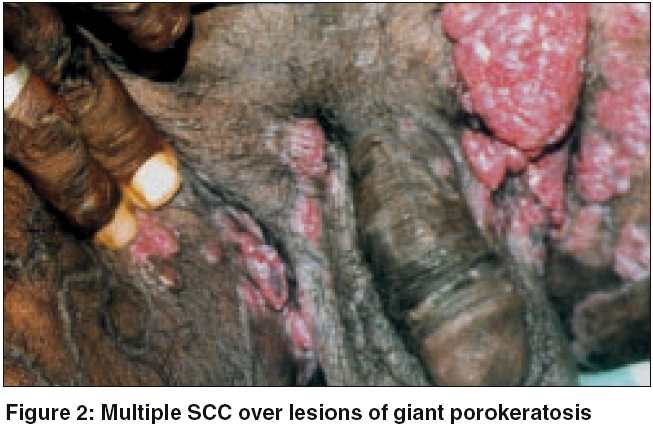
A 40-year-old farmer reported with asymptomatic skin lesions over the extremities, abdomen, groins, palms and soles for the past ten years. Since the last 6 months, he noted the appearance of multiple raised mildly itchy but painless growths over the pre-existing lesions in the groins. His family history was not suggestive of a similar disease. He denied an exposure to radiation and there was no history of high-risk sexual practices. No evidence of immunosuppression was apparent. On examination, numerous small well-defined hyperpigmented plaques, some of which were more than 10 cm in diameter, were seen. These giant lesions were present in the abdomen, thighs [Figure - 1] and soles. The involvement of the palms and soles was prominent. Individual plaques had an atrophic center with a prominent border that was traversed by a thread-like groove. In the inguinal region on both sides there were multiple erythematous papulonodular lesions of varying sizes, which bled on manipulation [Figure - 2]. The largest of them was 5cm x 3cm x 2cm. A few inguinal lymph nodes palpable were less than 0.5cm in size, soft to firm, not tender and mobile. No other lymph node was palpable. The rest of the cutaneous and systemic examination was normal.
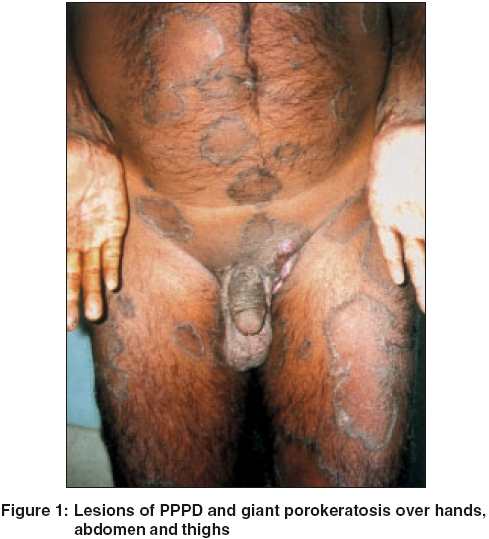
Routine blood tests, biochemical examinations, X-ray of the chest were normal. VDRL and ELISA for HIV were nonreactive. Histology from the edge of a flat lesion showed a parakeratotic column (cornoid lamella) in the epidermal invagination with absence of the underlying granular layer. A non-specific perivascular infiltrate of chronic inflammatory cells was seen in the dermis. These findings were consistent with porokeratosis. Fine needle aspiration and cytology (FNAC) from two contralateral inguinal nodules showed atypical cells and histopathology revealed features of a well-differentiated SCC in both [Figure - 3]. Ultrasonography of the abdomen showed no organomegaly, lymph node enlargement or any evidence of metastasis. A CT-guided FNAC of the inguinal and femoral lymph nodes showed reactive hyperplasia without any cellular atypia. The diagnosis of multicentric SCC arising from PPPD and giant porokeratosis was reached.
Discussion
Porokeratosis, first described by Mibelli in 1893, is a disorder of keratinization with an autosomal dominant inheritance and predilection for the male sex.[3] Mucous membrane lesions in the mouth, nose, glans penis and conjunctiva are rarely seen in PM, and PPPD.[4] Coexistence of different variants of porokeratosis may also occur.[5] Malignant degeneration has been found in all forms with a reported incidence of 7.5-11%.[1]
Our case had a combination of PPPD (which usually shows small lesions) and giant porokeratosis with SCC. A coexistence of disseminated superficial and giant porokeratosis with SCC has been reported in Indian literature[6] but the unique feature in our patient is the multicentric nature of the malignancy. There are references of multiple SCCs complicating LP and PPPD in foreign literature and Japanese patients are statistically more prone to develop them. [7],[8],[9] Fatal metastatic SCC can rarely occur in immunosuppressed patients with porokeratosis. Rongioletti et al[10] reported a case of disseminated porokeratosis in whom bowenoid lesion and SCC developed in a sequential pattern and metastasis proved to be fatal. They also found an over-expression of p53 protein in the keratinocytes near the cornoid lamella in a porokeratotic lesion and throughout the epidermis in a bowenoid lesion. So a functional aberration in this particular protein is the key-factor in the malignant transformation. Other proteins possibly involved are psi-3, cytokeratin, fillagrin and involucrin.[9]
Successful therapy has been tried with topical 5-flurouracil in PM, LP, as well as DSP and DSAP. Oral retinoids in the form of etretinate has been used to treat different forms of porokeratosis with conflicting results. Even though they have improved cytological atypia and halted carcinogenesis, the probability of relapse on discontinuation is a matter of concern.[8],[9] We tried excision of the tumours in multiple sittings followed by oral etretinate therapy 50mg/day, but the patient was lost to follow-up after about four months.
| 1. |
Spencer LV. Porokeratosis. www.emedicine. com/derm/topic342htm. Last accessed 31-08-2004.
[Google Scholar]
|
| 2. |
Griffiths WA, Judge MR, Leigh IM. Disorders of keratinization. In: Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Rook/ Wilkinson/ Ebling Textbook of Dermatology. 6th ed. Oxford: Blackwell Science; 1998. p. 1552-4.
[Google Scholar]
|
| 3. |
Pavithran K. Disorders of keratinization. In: Valia RG, Valia AR, editors. IADVL Textbook and Atlas of Dermatology. 2nd ed. Mumbai: Bhalani Publishing House; 2001. p. 805-6.
[Google Scholar]
|
| 4. |
Gangopadhyay AK. Porokeratosis palmaris plantaris et disseminata with a mucous membrane lesion. Indian J Dermatol Venereol Leprol 2000;4:205-6
[Google Scholar]
|
| 5. |
Kaur S, Thami GP, Mohan H, Kanwar AJ. Co-existence of variants of porokeratosis: a case report and review of literature. J Dermatol 2002;29:305-9.
[Google Scholar]
|
| 6. |
Hanumanthayya K, Nagari S, Tophakhane R, Rathor R. Coexistence of disseminated superficial and giant porokeratosis of Mibelli with squamous cell carcinoma. Indian J Dermatol Venereol Leprol 2003;69:296-7.
[Google Scholar]
|
| 7. |
Curnow P, Foley P, Baker C. Multiple squamous cell carcinoma complicating linear porokeratosis. Aust J Dermatol 2003;44:136-9.
[Google Scholar]
|
| 8. |
Seishima M, Izumi T, Oyama Z, Maeda M. Squamous cell carcinoma arising from lesions of porokeratosis palmaris plantaris disseminata. Eur J Dermatol 2000;10:478-80.
[Google Scholar]
|
| 9. |
Wolff-Schreiner EC. Porokeratosis. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's Dermatology in general medicine. 6th ed. New York: McGraw-Hill; 2003. p. 1796-801.
[Google Scholar]
|
| 10. |
Rongioletti F, Rebora A. Disseminated porokeratosis with fatal metastatic squamous cell carcinoma, an additional case of 'malignant disseminated porokeratosis'. Am J Dermatopathol 2002;24:144-8.
[Google Scholar]
|
Fulltext Views
2,430
PDF downloads
2,546





