Translate this page into:
Multiple adenoid basal cell carcinoma: An uncommon presentation
2 Department of Pathology, Cancer Institute, Adyar (W.I.A.), Chennai, Tamil Nadu, India
Correspondence Address:
Anand Raja
Department of Surgical Oncology, Cancer Institute, Adyar (W.I.A.), Chennai - 600 020, Tamil Nadu
India
| How to cite this article: Agarwal A, Raja A, Mahalingam S, Murhekar K. Multiple adenoid basal cell carcinoma: An uncommon presentation. Indian J Dermatol Venereol Leprol 2019;85:393-396 |
Abstract
Basal cell carcinoma (BCC) is the most common malignant skin tumor which occurs more frequently over the sun exposed parts of body. Its adenoid variant is a rare histological subtype. We report a case of multiple adenoid basal cell carcinomas at unusual sites in a middle-aged male patient.
Introduction
Basal cell carcinoma (BCC) is the most common cutaneous malignancy in humans.[1] According to Madras Metropolitan Tumor Registry data the crude incidence rate of BCC is 0.3/100,000 [unpublished data from Cancer Institute Adyar (W.I.A.)]. The most common presentation of BCC is a solitary nodular lesion or ulcer with rolled out margins over the sun exposed parts of the head and neck region in fair-skinned individuals.[2],[3] We present a rare case of multiple adenoid BCCs at unusual sites, involving both photoexposed and photoprotected sites, occurring in a dark skinned individual.
Case Report
A 55-year-old male agriculturist presented with a painless ulcerated swelling over his left arm, a proliferative pigmented swelling near the left elbow and another swelling in lumber region for the last 3 years. Initially these swellings were painless, however they gradually increased in size to become ulcerated and painful. There was no history suggestive of exposure to arsenic or family history of malignancy. The patient was immunocompetent and HIV sero negative. Physical examination was unremarkable except for presence of coarse facies. His skin type was Fitzpatrick Type V. Dermatological examination revealed two swellings in the left upper limb; the proximal one measuring 8 × 6 cm, situated over the deltoid region, showed ulceration at the summit and induration of the surrounding skin without infiltration of the underlying muscles [Figure - 1] and [Figure - 2]. The distal swelling over the lateral surface of the left elbow was 9 × 7 cm with black pigmentation, ulceration, and everted margins [Figure - 3]. The underlying muscles were not infiltrated and elbow movements were normal. The swelling over the back was 4 × 3 cm with surface ulceration and everted margins without infiltration of the underlying muscles [Figure - 4]. Multiple subcutaneous lipomas were noted in the abdominal wall. Axillary nodes were not enlarged. Patient had normal intellect. Nevoid BCC syndrome was ruled out clinically as our case developed BCC in the early fifth decade of life and there was absence of jaw cysts, skeletal anomalies and falx calcification on imaging. Biopsy from all the lesions showed tumor arising from the basal layer of epidermis with cells arranged in sheets, island and trabecular pattern with palisading nuclei [Figure - 5]. Interspersed microcystic spaces were present with melanin pigment incontinence. Immunohistochemistry (IHC) was strongly positive for Bcl 2 [Figure - 6]. Strong nuclear positivity for p53 and Ki 67 was seen. CK5/6 and HMWK were positive and c-Kit was positive only in the outermost layers of the cells. The patient underwent wide excisional biopsy of all the tumors. Histopathological examination confirmed the diagnosis of adenoid variant of basal cell carcinoma. Patient has been advised regular follow-up. No evidence of recurrence has been been noted 14 months post surgery.
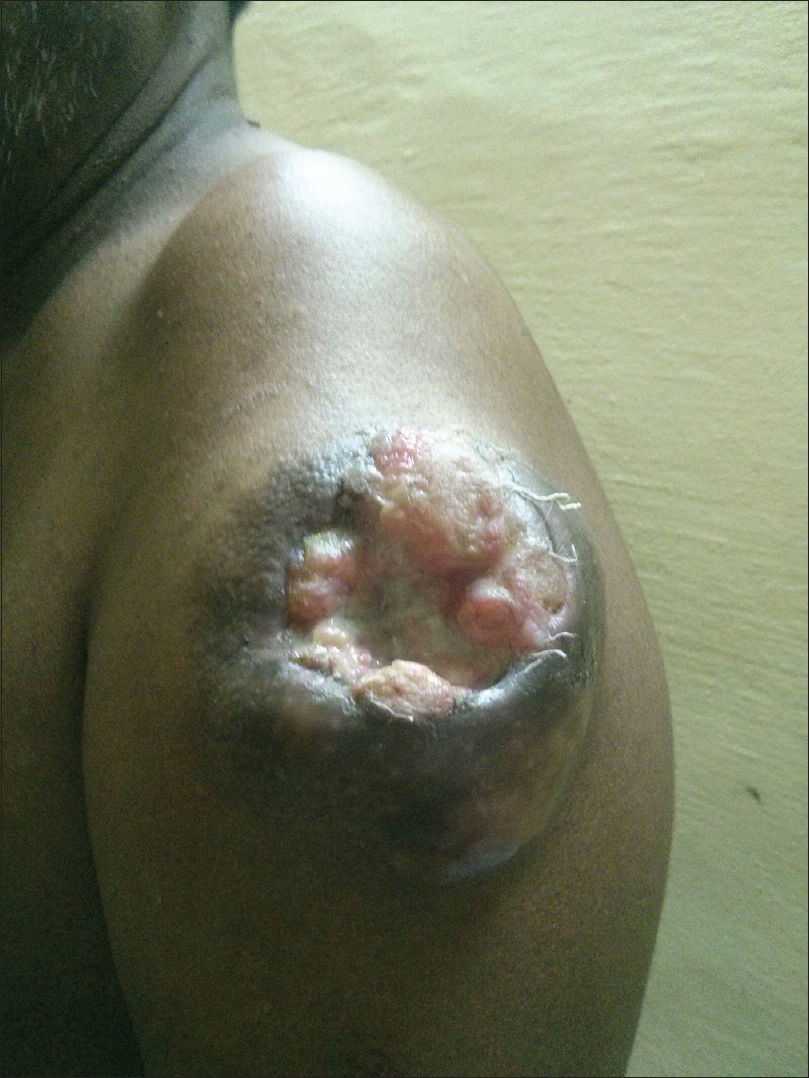 |
| Figure 1:Tumour with central ulceration and necrotic slough, seen over the deltoid region of the left arm |
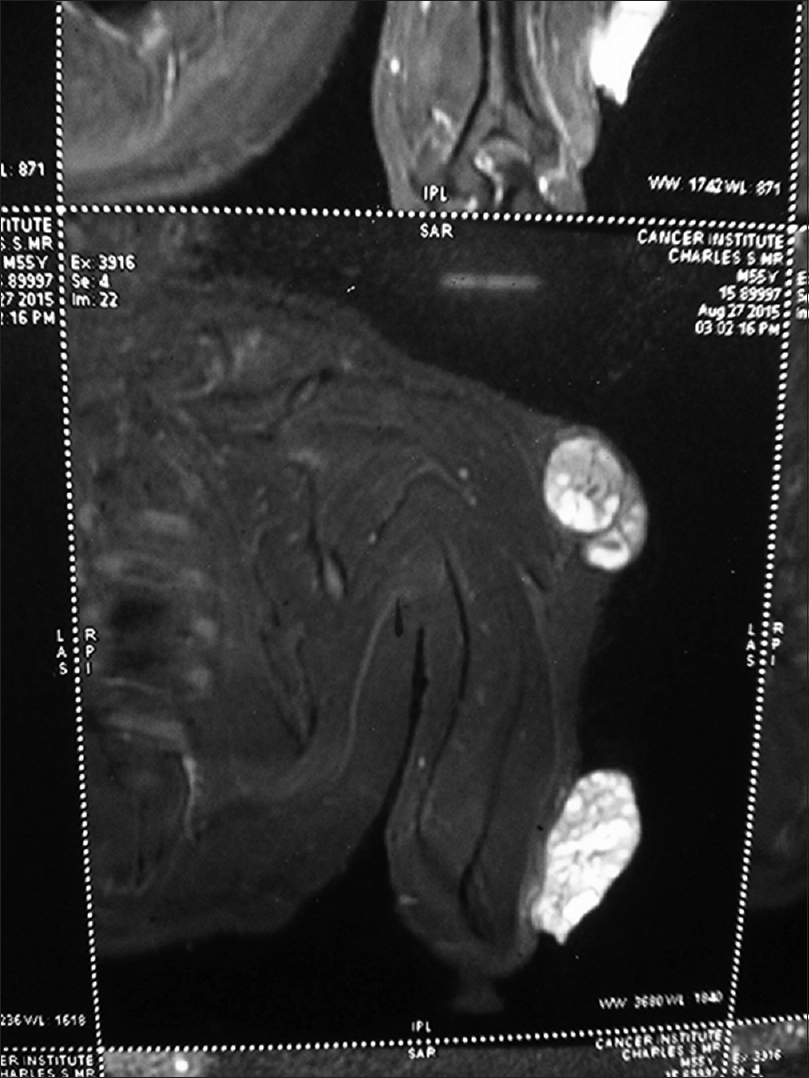 |
| Figure 2: Coronal short tau inversion recovery magnetic resonance images of the left arm lesions showing tumors with heterogenous enhancement and no infiltration of the underlying muscles |
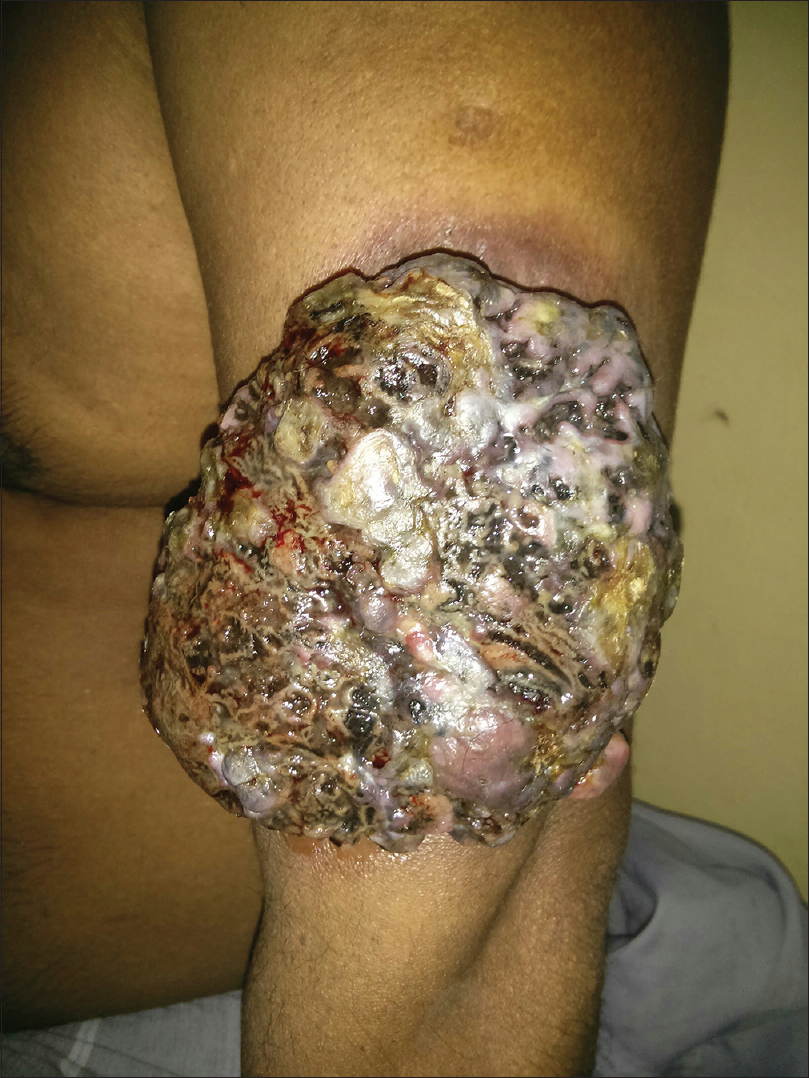 |
| Figure 3: Ulceroproliferative growth over the left elbow with a variegated pigmentation on surface |
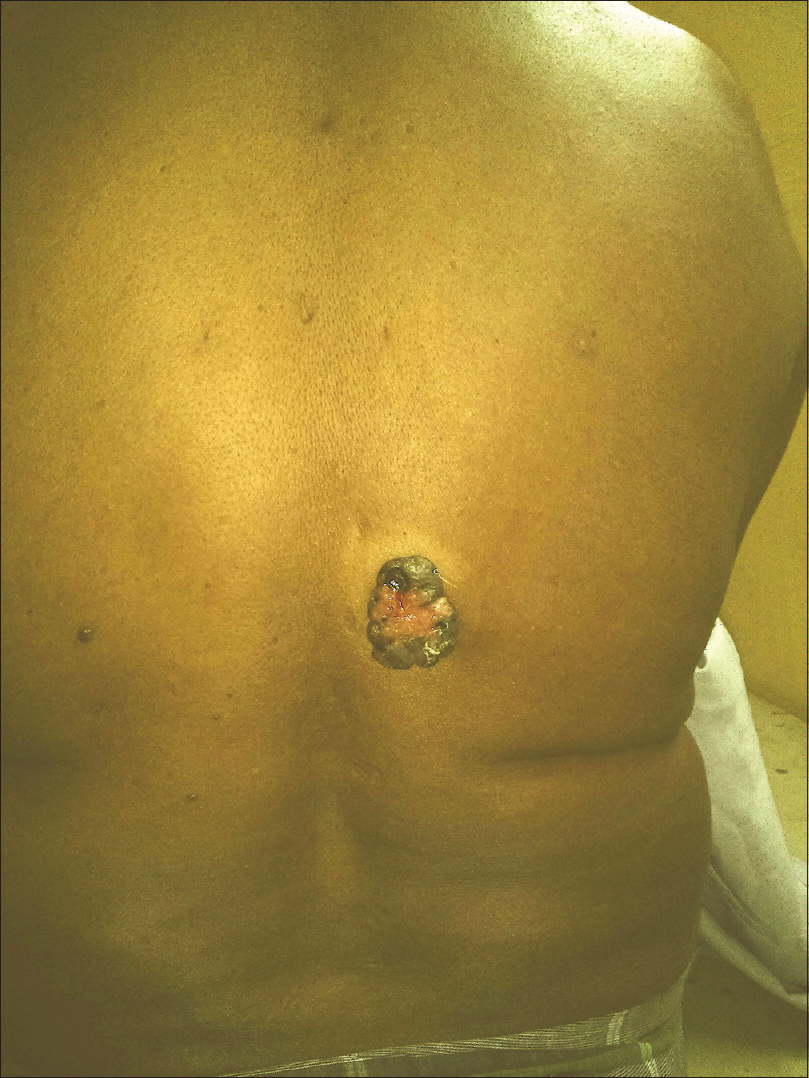 |
| Figure 4: Nodular growth with everted edges, peripherally pigmented and central erosion, present on the back |
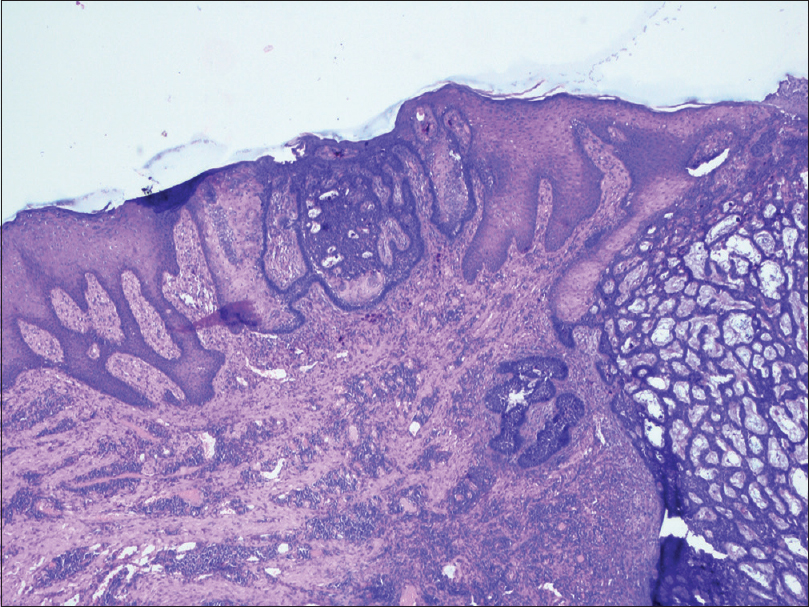 |
| Figure 5: HPE showing basaloid tumour cells extending from the epidermis downwards in a trabecular pattern. (×40) |
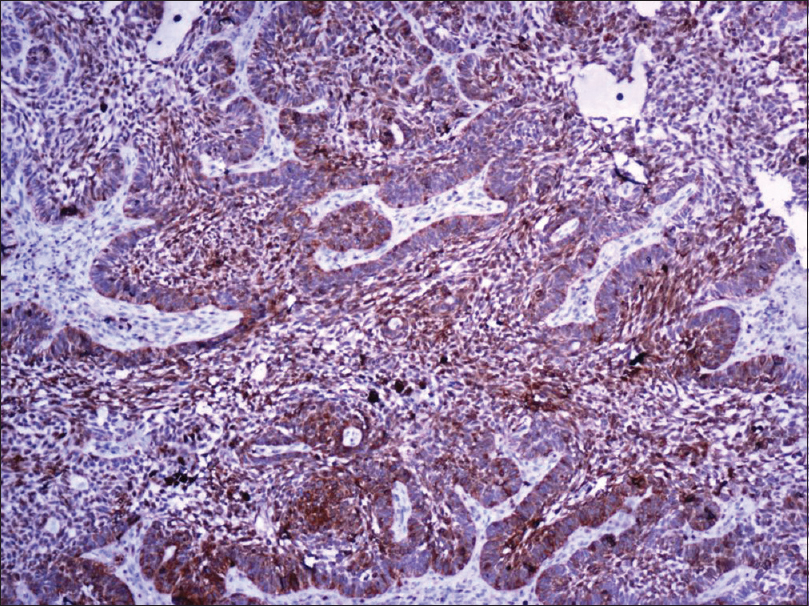 |
| Figure 6: Strong Bcl 2 immunostaining of the tumor cells (×400) |
Discussion
Basal cell carcinoma is the most common malignant skin tumor.[1] It accounts for approximately 75% of all nonmelanoma skin cancers (NMSC) and 25% of all cancers.[2] The tumor cells cytologically resemble normal epidermal basal cells, and it was believed that these are precursor tumor cells. BCC is now being increasingly recognized as a primitive adnexal tumor in light of the accumulating immunohistochemical evidence.[4],[5] Epidemiological data indicates that overall incidence is increasing worldwide roughly by 3–10% per year.[6] The various etiological factors implicated in the pathogenesis of this malignancy include exposure to sun light, arsenic ingestion, X-ray exposure, skin injury, chickenpox scars, tattoos, hair transplantation scars, and immunesuppression.[7],[8] It can also occur in the sunprotected parts of the body such as lower limbs and axilla.[7],[9] Multiple BCCs are usually associated with genodermatoses such as Basal cell nevus syndrome, Bazex syndrome, Rombo's syndrome and McKuisck syndrome, or immunocompromised mileu such as organ transplant recipients.[10] In case of multiple presentation phenotype, glutathione S-transferase (GSTM1, GSTT1) and cytochrome P450 (CYP2D6, CYP1A1) polymorphisms have been found to influence tumor numbers and accrual.[11] Relative tumor density index (RTD) arbitrarily defines the unusual sites of of BCC with palms, soles, axilla, groin, genitalia, intertrigenous areas, and glutei being some of the commonly reported unusual sites.[12],[13]
Basal cell carcinoma can have varied morphological appearances. Nodular variant is most common and appears as a pearly pink or flesh-colored papule with telangiectasia and rolled out borders. The superficial variant presents as a reddish plaque with variegated pigmentation and spreading margins. The more aggressive infiltrative or morhoeaform variant present as a depressed scarifirom plaque.[3] Histologically also BCC has many variants, of which the indolent-growth variants include superficial and nodular BCC. The aggressive growth tumors are infiltrative BCC, metatypical BCC, and morpheiform or sclerosing BCC.[14] The common types include nodular, superficial, and micronodular variants.[15] Rare morphological variants constitute less than 10% of all BCCs.[16]
Adenoid type is a histological variant of BCC. It is considered a subtype of nodular BCC.[17] On microscopy, tumor cells form tubular gland-like structures with cells being arranged in intertwining lace-like pattern around connective tissue forming radial strands, stroma being mucoid.[3] Similar appearance was observed on the microscopic examination of all the three lesions in our patient thus clinching the diagnosis of adenoid type of BCC. The exact incidence of adenoid variant is not well known. Various studies report figures ranging from 1.3% to 20.91%.[3],[18],[19],[20] A review of these reports suggests that, unlike other histological variants, the adenoid variant has an equal propensity to involve sites other than the head and neck region.[21] The various sites reported include face, scalp, chin, forehead, back, axilla, and eyelid.[18],[22] Multiplicity, involvement of both sun exposed and the sun protected parts, and varied morphological appearance constitute some of the atypical features of our case. However, we could not conduct studies on molecular markers due to lack of resources. The presence of multiple lesions prompted us to search for a syndromic cause. Nevoid BCC syndrome was the closest differential diagnosis but the middle age at presentation and absence of reported phenotypes ruled out known genetic syndromes.[23]Cutaneous adenoid cystic carcinoma and primary apocrine carcinoma have also been considered as differentials. Cutaneous adenoid cystic carcinoma is a rare skin tumor which can closely mimic adenoid BCC on histology. Adenoid cystic carcinoma shows strong positivity for C-kit (our case showed focal C-KIT positivity).
Cutaneous apocrine carcinoma is another differential diagnosis. Morphologically, the tumor is composed of neoplastic glands in the dermis arranged in a predominantly cribriform pattern. The cells show apocrine differentiation in the form of large round to polygonal cells with abundant eosinophilic granular cytoplasm and sometimes vacuolated cytoplasm. Adjacent areas of the tumor may show hyperplastic apocrine glands. On morphology, our case failed to show apocrine differentiation.
We were unable to find any other report describing multiple adenoid BCC involving both sun exposed and sun protected areas with different morphological appearances. This case augments our knowledge about adenoid variant of BCC for which literature is sparse.
The sporadic occurrence of multiple BCCs at both sun exposed and sun protected parts of the body with the rare histopathological type i.e. the adenoid variant and varied morphological appearance of the lesion are unique features in the present case report.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med 2005;353:2262-9.
[Google Scholar]
|
| 2. |
Crowson AN. Basal cell carcinoma: Biology, morphology and clinical implications. Mod Pathol 2006;19 Suppl 2:S127-47.
[Google Scholar]
|
| 3. |
Stoica LE, Georgescu CV, Patrascu V, Radu CC, Tolea I, Mogoanta L. Basal cell carcinomas-clinical-evolutional and histopahotologic aspects. Curr Health Sci J 2009;35:228-33.
[Google Scholar]
|
| 4. |
Schirren CG, Rütten A, Kaudewitz P, Diaz C, McClain S, Burgdorf WH, et al. Trichoblastoma and basal cell carcinoma are neoplasms with follicular differentiation sharing the same profile of cytokeratin intermediate filaments. Am J Dermatopathol 1997;19:341-50.
[Google Scholar]
|
| 5. |
Yada K, Kashima K, Daa T, Kitano S, Fujiwara S, Yokoyama S, et al. Expression of CD10 in basal cell carcinoma. Am J Dermatopathol 2004;26:463-71.
[Google Scholar]
|
| 6. |
Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, Kerl H. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol 2007;157 Suppl 2:47-51.
[Google Scholar]
|
| 7. |
Black MM, Walkden VM. Basal cell carcinomatous changes on the lower leg: A possible association with chronic venous stasis. Histopathology 1983;7:219-27.
[Google Scholar]
|
| 8. |
Nogita T, Kamikawa T, Kawashima M. Significance of pre-existent conditions in basal cell carcinoma on the lower extremities. Int J Dermatol 1993;32:350-3.
[Google Scholar]
|
| 9. |
Cohen PR. Basal cell carcinoma of the axilla: Review of the world literature. Am J Clin Dermatol 2014;15:95-100.
[Google Scholar]
|
| 10. |
Tsai KY, Tsao H. The genetics of skin cancer. Am J Med Genet C Semin Med Genet 2004;131C:82-92.
[Google Scholar]
|
| 11. |
Yengi L, Inskip A, Gilford J, Alldersea J, Bailey L, Smith A, et al. Polymorphism at the glutathione S-transferase locus GSTM3: Interactions with cytochrome P450 and glutathione S-transferase genotypes as risk factors for multiple cutaneous basal cell carcinoma. Cancer Res 1996;56:1974-7.
[Google Scholar]
|
| 12. |
Bhagchandani L, Sanadi RE, Sattar S, Abbott RR. Basal cell carcinoma presenting as finger mass. A case report. Am J Clin Oncol 1995;18:176-9.
[Google Scholar]
|
| 13. |
Nagendra Naidu DV, Rajakumar V. Perianal basal cell carcinoma-an unusual site of occurrence. Indian J Dermatol 2010;55:178-80.
[Google Scholar]
|
| 14. |
Crowson AN, Magro CM, Kadin ME, Stranc M. Differential expression of the bcl-2 oncogene in human basal cell carcinoma. Hum Pathol 1996;27:355-9.
[Google Scholar]
|
| 15. |
Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol 1990;23:1118-26.
[Google Scholar]
|
| 16. |
Eibenschutz L, Colombo D, Catricalà C. Everolimus for compassionate use in multiple Basal cell carcinomas. Case Rep Dermatol Med 2013;2013:604301.
[Google Scholar]
|
| 17. |
Dandurand M, Petit T, Martel P, Guillot B, ANAES. Management of basal cell carcinoma in adults clinical practice guidelines. Eur J Dermatol 2006;16:394-401.
[Google Scholar]
|
| 18. |
Koyuncuer A. Histopathological evaluation of non-melanoma skin cancer. World J Surg Oncol 2014;12:159.
[Google Scholar]
|
| 19. |
Mateoiu C, Pirici A, Bogdan F. Immunohistochemical nuclear staining for p53, PCNA, ki-67 and bcl-2 in different histologic variants of basal cell carcinoma. Rom J Morphol Embryol 2011;52:315-9.
[Google Scholar]
|
| 20. |
Hussain I, Soni M, Khan BS, Khan MD. Basal cell carcinoma presentation, histopathological features and correlation with clinical behaviour. Pak J Ophthalmol 2011;27:3-7.
[Google Scholar]
|
| 21. |
Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: Rare histopathological variant at an unusual location. Indian J Dermatol 2013;58:159.
[Google Scholar]
|
| 22. |
Bastiaens MT, Hoefnagel JJ, Bruijn JA, Westendorp RG, Vermeer BJ, Bouwes Bavinck JN. Differences in age, site distribution, and sex between nodular and superficial basal cell carcinoma indicate different types of tumors. J Invest Dermatol 1998;110:880-4.
[Google Scholar]
|
| 23. |
Kimonis VE, Goldstein AM, Pastakia B, Yang ML, Kase R, DiGiovanna JJ, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet 1997;69:299-308.
[Google Scholar]
|
Fulltext Views
5,956
PDF downloads
1,761





