Translate this page into:
Mycobacterium indicus pranii vaccine immunoprophylaxis in anti-phenolic glycolipid-1-positive leprosy contacts – A pilot study from a tertiary care center in North India
-
Received: ,
Accepted: ,
How to cite this article: Kumaran MS, Narang T, Chabbra S, Ashraf R, Dogra S. Mycobacterium indicus pranii vaccine immunoprophylaxis in anti-phenolic glycolipid-1-positive leprosy contacts – A pilot study from a tertiary care center in North India. Indian J Dermatol Venereol Leprol 2022;88:47-50.
Abstract
Background:
Contacts of leprosy patients have an increased risk of infection with Mycobacterium leprae. Contact tracing and chemo- or immunoprophylaxis are important means of preventing leprosy transmission.
Aims:
We aimed to evaluate the efficacy of immunoprophylaxis with Mycobacterium indicus pranii vaccine in reducing anti-phenolic glycolipid-1 titers in household contacts of leprosy patients.
Methods:
This prospective single-center study was conducted in a tertiary care center in North India from January 2015 to December 2016. Contacts of leprosy patients (both paucibacillary and multibacillary) were screened for anti-phenolic glycolipid-1 antibodies with enzyme-linked immunosorbent assay. Those found positive were given immunoprophylaxis with a single dose of Mycobacterium indicus pranii vaccine, and anti-phenolic glycolipid-1 titers were evaluated at six and 12 months. All contacts were clinically followed for three years.
Results:
Of the 135 contacts of 98 leprosy patients that were screened, 128 were recruited. Seventeen of these contacts were positive for anti-phenolic glycolipid-1 antibodies and were given Mycobacterium indicus pranii vaccine. Two contacts were lost to follow-up. After immunoprophylaxis, anti-phenolic glycolipid-1 titers were negative in all patients at all intervals, and no contact developed any clinical signs or symptoms of leprosy during the three-year follow-up.
Limitations:
The small number of contacts studied, the short follow-up period and the absence of a control group were limitations of this study.
Dicussion:
We could not find any papers on natural decline of PGL 1 titres in contacts, although in leprosy patients, these titres may even increase after completion of treatment. However the titres do correlate with bacterial load (reference: Int J Lepr Other Mycobact Dis. 1998 Sep;66(3):356-64) so if the tires decrease or become negative it may be considered as an indirect evidence of bacillary clearance. Hence we may suggest the protective efficacy. Furthermore, as the editor mentioned, considering the small number of positive patients, a control group was not possible in the present pilot study, but such studies may be carried out in the future.
Conclusion:
Immunoprophylaxis with Mycobacterium indicus pranii vaccine is effective and safe in preventing disease in contacts of leprosy patients. However, these findings need to be replicated in larger studies.
Keywords
Mycobacterium indicus pranii
Immunoprophylaxis in leprosy
anti-phenolic glycolipid-1 antibodies
Plain Language Summary
Contacts of leprosy patients are at increased risk of getting infection. Interruption of leprosy transmission is necessary to achieve the goal of a leprosy free world. This study evaluates the preventive effect of Mycobacterium indicus pranii vaccine in contacts of leprosy patients. All anti-phenolic glycolipid-1-positive contacts (which implies exposure to leprosy bacteria) were administered Mycobacterium indicus pranii vaccine, and they became negative in the follow-up period.
Introduction
The implementation of multidrug therapy by the WHO and a focus on early diagnosis and treatment has resulted in a decrease in the global burden of leprosy, thus raising hopes of a leprosy free world. Prevention of leprosy in high-risk groups is an important strategy in reducing the prevalence of the disease.1
Clinical findings in the early stages of infection are often absent and hence serological tests, such as the phenolic glycolipid-1 enzyme-linked immunosorbent assay, are used to detect the presence of infection in contacts.2 Contacts of patients with lepromatous leprosy have a 3.8-fold higher risk of developing leprosy,3 but anti-phenolic glycolipid-1 antibody positive contacts have a six-fold higher risk of developing disease as compared to anti-phenolic glycolipid-1-negative contacts.2,4-7
Immunoprophylaxis of high-risk groups such as household or close contacts is an important strategy in controlling leprosy transmission.7 However, there are neither data, nor consensus, on who should be offered immunoprophylaxis. Markers to assess the efficacy of such interventions as immuno- or chemoprophylaxis early (rather than the usual ten–15 years necessary to observe a drop in leprosy incidence) are needed.
In this study, we explored the efficacy of Mycobacterium indicus pranii vaccine in the reduction of anti-phenolic glycolipid-1 titers in contacts of patients with leprosy.
Materials and Methods
This prospective study was conducted at the Postgraduate Institute of Medical Research, Chandigarh. Approval for this study was obtained from the Institute Ethics Committee.
Contacts of consecutively registered leprosy patients from January 2015 to December 2016 were enrolled. Contacts were classified as domiciliary (household contacts) or nondomiciliary (relatives and neighbors). Household contacts were defined as individuals who currently resided or had resided with the patient in the past five years. Only household contacts who shared the same household and kitchen were selected for study.
After obtaining informed consent, a thorough clinical examination was performed in all contacts and those with active disease were excluded from the study. The presence of a BCG scar was noted. The nature of contact and the relationship with patient was ascertained. Slit-skin smears and anti-phenolic glycolipid-1 enzyme-linked immunosorbent assay were performed in all contacts. Serum samples from 40 healthy individuals who had no contact with leprosy patients were used as negative controls for enzyme-linked immunosorbent assay tests.
All anti-phenolic glycolipid-1 enzyme-linked immunosorbent assay positive contacts were given 0.1 ml of Mycobacterium indicus pranii vaccine (Cadila Pharma, Ahmedabad, India) intradermally in divided doses over both the deltoids. Anti-phenolic glycolipid-1 enzyme-linked immunosorbent assay was repeated at six months and one year in vaccinated contacts. Follow-up evaluations of all contacts (both phenolic glycolipid-1 positive and negative) were performed every six months for three to assess for adverse events as well as for the development of leprosy. The mean and standard deviations were calculated.
Serological assessment (anti-PGL-1 ELISA-linked immunosorbent assay estimation): The detailed methodology is given in the appendix.
Results
We assessed 135 household contacts of 94 leprosy patients (M: F = 1.76:1, mean age 39.5 ± 13 years). The disease duration in the 94 patients ranged from 1.5 years to eight years and included 19 (20.2%) borderline tuberculoid, 24 (25.5%) borderline lepromatous, 49 (52.1%) lepromatous and 2 (2%) histoid leprosy patients [Figure 1 and Table 1]. Seven of the 135 contacts were excluded as they had active disease and the remaining 128 contacts selected for study comprised 25 contacts of paucibacillary and 103 contacts of multibacillary leprosy.

- Distribution of patients and contacts according to spectrum
| S. No | Patient age | Sex | Spectrum | Duration | BI | MI (%) | Reactions | Relapse | Contact age | Sex | Relation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | F | BT | 1 year | 0 | 0 | Type 1 | None | 16 | F | Daughter |
| 2 | 31 | M | LL | 1 year | 3 | 0 | Type 2 | Yes | 25 | F | Wife |
| 3 | 50 | F | BL | 1 year | 5 | 15 | None | None | 17 | M | Son |
| 4 | 35 | F | BL | 7 months | 5 | 3 | None | None | 15 | M | Son |
| 5 | 24 | M | BT | 5 months | 0 | 0 | None | None | 27 | M | Brother |
| 6 | 42 | M | BT | 3 months | 0 | 0 | None | None | 30 | F | Wife |
| 7 | 40 | F | BT | 1 year | 0 | 0 | Type 1 | None | 20 | F | Daughter |
| 8 | 40 | F | BT(MB) | 1 year | 0 | 0 | Type 1 | None | 18 | M | Son |
| 9 | 53 | F | BL | 1 year | 4 | 4 | Type 1 | None | 25 | F | Daughter |
| 10 | 25 | M | LL | 3 years | 5 | 4 | Type 2 | None | 23 | F | Wife |
| 11 | 37 | M | LL | 1 year | 0 | 0 | None | Yes | 40 | F | Wife |
| 12 | 48 | M | LL | 2 years | 4 | 2 | Type 2 | None | 50 | M | Brother |
| 13 | 65 | M | Histoid | 2 years | 4 | 2 | None | None | 35 | F | Daughter |
| 14 | 38 | F | LL | 4 years | 6 | 3 | Type 2 | None | 42 | M | Husband |
| 15 | 55 | M | BT (MB) | 2 years | 0 | 0 | None | None | 20 | M | Brother |
| 16 | 35 | M | LL | 5 years | 5 | 4 | None | None | 30 | F | Wife |
| 17 | 56 | M | Histoid | 2 years | 3 | 2 | None | None | 30 | F | Daughter |
BI: Bacteriological index, MI: Morphological index, F: Female, M: Male, BT: Borderline tuberculoid, LL: Lepromatous, BL: Borderline lepromatous, MB: Multibacillary
Patient 11 had clinically early diffuse LL whose BI/MI was negative on SSS, but histopathology was consistent with multibacillary spectrum, showing positive FiteFaraco staining.
Patients 8 and 15 were classified to be multibacillary based on the number of lesions (>5) which belonged to BT spectrum even though SSS was negative for AFB.
All 128 recruited contacts (M: F = 1.66:1, mean age 27 ± 15 years) had a BCG scar and no signs or symptoms of leprosy. The duration of contact with the leprosy patients varied from five years to 48 years. The majority (66, 51.5%) were contacts of lepromatous patients, while 30 (23.4%), 27 (21%) and five (3.9%) were contacts of borderline tuberculoid, borderline lepromatous and histoid leprosy patients, respectively [Figure 2].
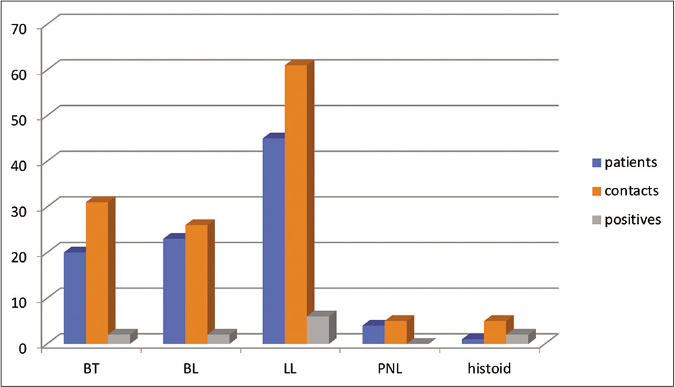
- Representation of leprosy spectrum among patients, contacts and those positive for phenolic glycolipid-1
Only 17 (13.3%) of the contacts were anti-phenolic glycolipid-1 antibody positive – six each were contacts of borderline tuberculoid and lepromatous, three were contacts of borderline lepromatous and two of histoid leprosy [Figure 2]. The titres were higher in contacts of male leprosy patients having multibacillary disease. All anti-phenolic glycolipid-1 antibody positive contacts were treated with Mycobacterium indicus pranii vaccine. Two contacts were lost to follow-up due to personal reasons and the remaining 15 contacts completed the total study period. Anti-phenolic glycolipid-1 titers were negative at six months and one year in all 15 contacts [Figure 3]. At completion of study, none of the contacts (anti-phenolic glycolipid-1 positive or negative) had any clinical signs of leprosy. Injection site reactions as an ulcerated tender nodule was observed in 4/15 contacts. No other significant adverse events were encountered.
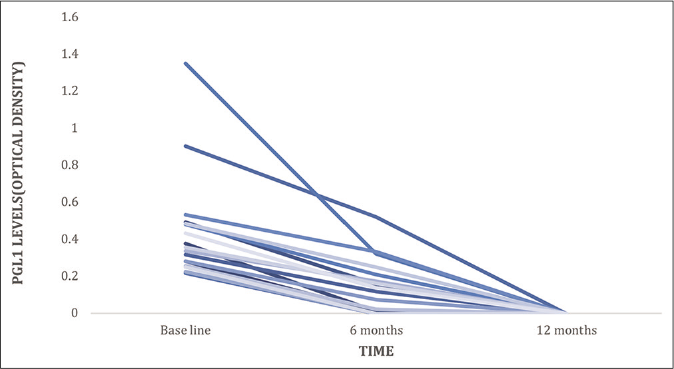
- Fall in phenolic glycolipid-1 antibody titers among contacts after vaccination
Discussion
Leprosy is a complex disease manifesting a wide spectrum of clinical manifestations. India has the largest burden of leprosy patients with more than 126,000 new infections detected in 2017–2018,8 but actual numbers may be higher. India, Indonesia and Brazil together account for approximately 80% of the total leprosy cases of the world and there is an urgent need to halt the transmission of leprosy.
With disease rates still high in the community, it is imperative to target leprosy contacts, especially those of multibacillary patients, as a preventive strategy for leprosy control. Araujo et al.,9 in their study, observed that the majority of new cases among household contacts appeared by the first year of follow-up, emphasizing not only the importance of the initial examination but also close monitoring of household contacts for at least a year or more. Expectedly, most household contacts contracting leprosy were contacts of multibacillary leprosy patients, highlighting the need to especially monitor household contacts of multibacillary cases. BCG vaccination has been a prevention strategy against leprosy since 1960,10 and it has been shown to offer protection to the tune of 50%, especially in contacts of multibacillary patients.11 Vaccine trials from Venezuela and Malaysia measuring the outcome of BCG alone or in combination with killed Mycobacterium leprae demonstrated a decrease in leprosy incidence across all ages.11 Carvelho et al.12 observed that BCG vaccination of household contacts leads to a significant increase in memory CD4 and CD8 T cell to Mycobacterium leprae antigens at six months, suggesting a specific protective response. In a field trial of Mycobacterium indicus pranii vaccine in leprosy contacts in Uttar Pradesh, India, a protective efficacy of 69% and 59% for three and five years, respectively, was demonstrated. However, serological correlation was not attempted in this study.13
In the present study, 17 (13.3%) of the 135 household contacts demonstrated anti-phenolic glycolipid-1 antibodies in blood and were given Mycobacterium indicus pranii vaccine immunoprophylaxis. Fifteen contacts, who were given immunoprophylaxis, completed the study and were negative for anti-phenolic glycolipid-1 antibodies at six months and one year and none of these developed signs or symptoms of leprosy during the three years of follow-up. Though we were unable to find any studies commenting on natural decline of PGL 1 titres in contacts, it has been noted that the titres do correlate positively with bacterial load.14 Hence, if the titres decrease or become negative, it may be considered as an indirect evidence of bacillary clearance.
Limitations
The major limitation of our study was the small number of anti-phenolic glycolipid-1-positive contacts, the short follow-up period and the absence of control group. Another limitation was the lack of an untreated control group of anti-PGL +ve contacts, left untreated to find out how many became negative on their own in the follow-up period.
Conclusion
Immunotherapy with Mycobacterium indicus pranii vaccine resulted in complete clearance of anti-phenolic glycolipid-1 antibodies in contacts that might otherwise have developed leprosy. Immunoprophylaxis was safe and without any serious side effects.
Appendix
Serological assessment (anti-phenolic glycolipid-1 enzyme-linked immunosorbent assay estimation):
Whole blood (three milliliter) without anticoagulants was obtained from household contacts and controls. Serum was separated and stored at –200°C until needed.
Phenolic glycolipid-1 antigen (ND-O-BSA, phenolic glycolipid-1-based glycoconjugate of bovine serum albumin, catalog no. NR-19346; BEI resources, USA. ND-O-BSA) was reconstituted by dissolving one milligram in one milliliter of deionized water to make a final concentration of one milligram/ milliliter. Then, the coating buffer (carbonate-bicarbonate buffer) with a pH of 9.6 for coating the plate was prepared. The enzyme-linked immunosorbent assay plate (Tarson, India) was coated with the antigen in coating buffer at a concentration of 100 ng/well. The plate was incubated overnight at 37°C in a humidified chamber and then washed for two minutes with phosphate buffer saline four times. Bovine serum albumin-phosphate buffer saline 2% (Sigma)(200 ul/well) was then added to the plates and incubated at 37°C for one and half hours in a humidified chamber. The plate was washed again with phosphate buffer saline. The serum sample diluted with 1% bovine serum albumin (100 ul/well) was then added to the plate and incubated at 37°C in a humidified chamber for two hours. The plates were then washed with phosphate buffer saline-Tween 20 (T). Horseradish peroxidase-conjugated anti-human IgM (Sigma) (1:4000 dilution, 100 ul) was added to each well and the plate was again incubated at 37°C in a humidified chamber for 90 min. After again washing the plate, 100 ul conjugate o-phenylenediamine (Sigma) was added to each well and the plate was then incubated for 20 min in the dark. The reaction was stopped with 3 N hydrochloric acid and optical densities were read at a wavelength of 490/630 nm.
Optical density values of different dilutions of pooled sera (standards) were then plotted to get standard graph. A cutoff value (0.217892) has already been established in our laboratory using sera from healthy subjects. Optical densities above this cutoff value were considered positive and below were considered negative for anti-phenolic glycolipid-1.
The standard was prepared from pooled sera from ten lepromatous leprosy patients showing high antibody titer against the antigen to be tested, at dilution of 1:200–1:6400 with bovine serum albumin-phosphate buffer saline. Appropriate negative and positive controls were also run in parallel. Phenolic glycolipid-1-positive serum from lepromatous leprosy patients was taken as a positive control and phenolic glycolipid-1-negative sera from a healthy control were taken as a negative control.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Leprosy among patient contacts: A multilevel study of risk factors. PLoS Negl Trop Dis. 2011;5:e1013.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of Mycobacterium leprae DNA in the skin lesions of leprosy patients may be affected by amplicon size. Arch Dermatol Res. 2007;299:267-71.
- [CrossRef] [PubMed] [Google Scholar]
- Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts. Clin Vaccine Immunol. 2008;15:101-5.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-PGL-1 positivity as a risk marker for the development of leprosy among contacts of leprosy cases: Systematic review and meta-anlaysis. PLoS Negl Trop Dis. 2016;10:e0004703.
- [CrossRef] [PubMed] [Google Scholar]
- Serological activity of a characteristic phenoloc glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol. 1983;52:271-9.
- [Google Scholar]
- Factors associated with anti-phenolic glycolipid-1 seropositivity among the house hold contacts of leprosy cases. BMC Infect Dis. 2015;15:219.
- [CrossRef] [PubMed] [Google Scholar]
- Combination chemoprophylaxis and immunoprophylaxis in reducing the incidence of leprosy. Risk Manag Healthc Policy. 2016;9:43-53.
- [CrossRef] [PubMed] [Google Scholar]
- Unveiling healthy carriers and subclinical infections among household contacts of leprosy patients who play potential roles in the disease chain of transmission. Mem Inst Oswaldo Cruz. 2012;107:55-9.
- [CrossRef] [PubMed] [Google Scholar]
- BCG vaccination and Leprosy protection: Review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9:209-22.
- [CrossRef] [PubMed] [Google Scholar]
- Interruption of persistent exposure to leprosy combined or not with recent BCG vaccination enhances the response to Mycobacterium leprae specific antigens. PLoS Negl Trop Dis. 2017;11:e0005560.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127-43.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of anti-PGL-I as a prognostic marker of leprosy reaction. Int J Lepr Other Mycobact Dis. 1998;66:356-64.
- [Google Scholar]






