Translate this page into:
Mycophenolate mofetil as adjuvant in pemphigus vulgaris
Correspondence Address:
Nilendu Sarma
P. N. Colony, Sapuipara, Bally, Howrah - 711227
India
| How to cite this article: Sarma N, Ghosh S. Mycophenolate mofetil as adjuvant in pemphigus vulgaris. Indian J Dermatol Venereol Leprol 2007;73:348-350 |
Abstract
Pemphigus vulgaris (PV) is a life threatening autoimmune blistering disease of skin and mucous membranes. Advent of systemic steroids has greatly reduced the mortality rate. However, steroids and adjuvant immunosuppressive therapy are nowadays frequent contributory agents of morbidity and mortality of PV. Mycophenolate mofetil (MMF) has been reported to be an effective adjuvant to systemic steroids. It helps in increasing the immunosuppressive effect and minimizing the toxicities by steroid sparing effect. However, its efficacy in refractory cases of PV is not well documented. The lowest possible dose with satisfactory therapeutic efficacy and least side effects is known. We used MMF 1 g/day and systemic steroids in 3 Indian patients with pemphigus vulgaris who were resistant to systemic steroid monotherapy or combination treatment with azathioprine. In our experience, MMF offers an effective adjuvant with minimal side-effects in the treatment of resistant PV.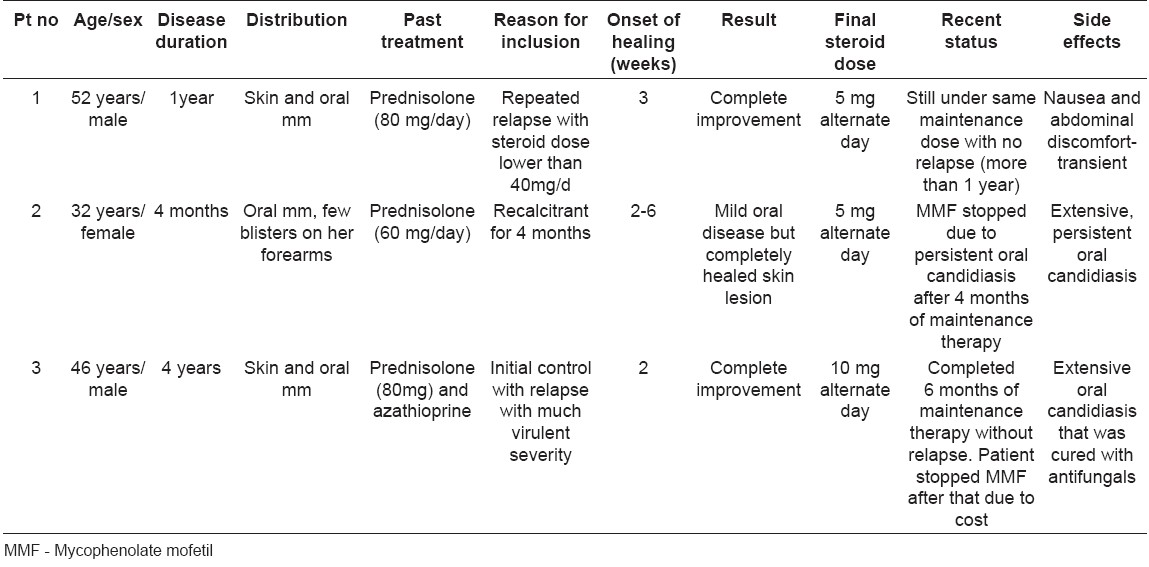

Introduction
Pemphigus vulgaris (PV) is a life threatening autoimmune blistering disease of skin and mucous membrane. Adjuvant immunosuppressive therapy has dramatically altered the prognosis of pemphigus vulgaris patients in recent decades. However, pemphigus still continues to be associated with significant morbidity and mortality, frequently due to the therapy itself. [1],[2] The immunosuppressive agents, such as mycophenolate mofetil, azathioprine, and cyclophosphamide have steroid sparing effects, decrease the incidence of side effects of therapy and may increase the number of remissions. [1],[3],[4] Mycophenolate mofetil (MMF), a morpholino ester of mycophenolic acid, acts by inhibiting de novo purine synthesis. Cells replicating on de novo purine synthesis rather than the purine salvage pathway are preferentially affected. Therefore, the proliferative responses of T and B-lymphocytes, which lack the purine salvage pathway, are blocked. It also leads to decreased levels of immunoglobulins and affects delayed type hypersensitivity responses. MMF in particular, among the adjuvant immunosuppressants used in PV, has been shown to have a rapid effect in lowering pemphigus antibody titers and to decrease the disease activity.
It has been found to be effective even in patients whose disease is unresponsive to azathioprine. [4] From India, however, no published documentation of use of MMF in pemphigus vulgaris is available up till now to the best of our knowledge. We tried MMF as steroid adjuvant in three cases of refractory PV.
Case Reports
Out of the total 3 patients with PV, two were male and one female. In all the cases, the disease was confirmed by routine histopathology. Direct immunofluorescence (DIF) from skin as well as oral lesions was done in patient no. 2 [Table - 1].
The original steriod dose and indications for intiating adjuvant MMF therapy are mentioned in [Table - 1].
None of these patients had history of MMF hypersensitivity or were suffering from peptic ulcer disease, hepatic or renal disease or were on concomitant azathioprine or cholestyramine therapy. Investigations including complete hemogram, liver and renal function test were done at the onset and repeated every 3 weeks. Written consent was obtained from each patient after explanation in detail regarding the disease as well as possible side effects of the drugs. MMF was given in the dose of 1000 mg/day from day one.
Case 1
A 52-year-old man had PV on his skin and oral mucosa for the last 1 year when he presented to us. His disease was controlled with prednisolone (80 mg/day) but relapsed regularly whenever the steroid was tapered to 40 mg/day. He was given oral prednisolone (60 mg/day) and MMF (1000 mg/day). The disease was controlled within 3 weeks at which point the dose of prednisolone was gradually reduced. Initially prednisolone was reduced by 10 mg every week till the daily dose reached was 20 mg/day. Then the dose was reduced alternate week till a dose of 20 mg on alternate day was reached. Then the dose reduction was even slower till a final dose of 5 mg on alternate day was reached. The final dose is being maintained till date. There was no relapse during the entire period of therapy. Only mild nausea and abdominal discomfort was reported at the onset of the therapy but improved with continuation of the therapy.
Case 2
A 32-year-old lady had oral ulcers with a few blisters on her forearms. PV was diagnosed with the help of routine histopathology and DIF from both her oral mucosa and skin vesicle. Her disease was not controlled with systemic steroids given for 4 months at the time she presented to us. We put her on oral prednisolone (60 mg/day) and 1000 mg of MMF. Improvement started in skin lesions within 2 weeks but the oral mucosal lesions took longer time (6 weeks) to heal. Thereafter steroid dose reduction was attempted in the same schedule as in Case 1. A few new oral mucosal lesions appeared at a dose of 20 mg on alternate day. However, they were mild, and dose reduction continued. At the dose of 5 mg on alternate day, there were no skin lesions while oral erosions persisted in a mild form. After 2 weeks from the onset of therapy, her oral mucosa got covered with extensive candidiasis for which she had to take oral fluconazole 50 mg daily for 3 weeks followed by topical clotrimazole oral paint regularly throughout the treatment. Oral candidiasis, however, continued in spite of the therapy. MMF was stopped after 4 month of maintenance therapy to control oral candidiasis.
Case 3
A 46-year-old male with PV was controlled with oral prednisolone and azathioprine. His disease relapsed with more severity as he stopped treatment prematurely. Oral prednisolone (80 mg/day) and MMF (1000 mg/day) was started. New blisters stopped appearing within 2 weeks. Steroid dose was reduced in the same fashion as for Case1. A final steroid dose of 10 mg on alternate day was achieved with no relapse and maintained for 6 months.
This patient also developed extensive oropharyngeal candidiasis 2 weeks after starting MMF but was cleared with oral fluconazole 100 mg daily for 14 days followed by topical clotrimazole troche.
Discussion
Systemic corticosteroids is the most potent therapeutic option for pemphigus vulgaris. With its use, mortality rate has come down significantly from 60-90% [5] to below 10%. [2] Most of the present mortality is due to treatment-induced toxicities as all drugs used are potent immunosuppressants. [1],[2] Judicious use of adjuvants can reduce this rate significantly. [6] Adjuvants help to lower initial steroid dose without relapse, faster reduction of steroid dose as well as much less final maintenance dose, thus minimizing steroid induced potential systemic toxicities.
MMF has been successfully used in PV as monotherapy [7] or as adjuvants. [8],[9] There are however, not many studies that have attempted to evaluate its efficacy exclusively in refractory cases of pemphigus vulgaris. [10] Adverse effects of MMF are carcinogenicity (controversial), gastrointestinal disorders (most common), genitourinary complaints, increased incidence of viral or bacterial infection and neurological symptoms. [11] Absolute contraindications of MMF are pregnancy and drug hypersensitivity; relative contraindications include lactation, peptic ulcer disease, hepatic or renal disease and concomitant azathioprine or cholestyramine therapy.
The objective of our therapy was to use the lowest possible dose of steroid for maintenance effect, early withdrawal and prolong steroid free periods with the help of use of low dose MMF. Our preliminary study of these three cases treated with oral corticosteroids along with MMF as adjuvant revealed that MMF is a clinically effective immuno-suppressant with early onset of action in PV cases.
Nousari et al . reported that immuno-bullous diseases require higher dose of MMF than other inflammatory diseases. [11] Powel et al. found in their study that doses higher than 2 gm/day had no therapeutic benefit and at the same time were associated with more side effects especially leucopenia. They used different doses of MMF with an average dose of 2.5 g/day. Although, we did not compare different doses of MMF, our experience suggests that lower dose of 1 g/day is also effective and is not associated with any significant side effects.
Opportunistic infections are common side effects of all immuno-suppressants including MMF. However extensive oral candidiasis as seen in two of our cases was not a frequently reported event following MMF therapy. One major drawback of the drug is its high cost, which remains an important factor to be considered in developing countries like ours, especially when MMF is to be continued for an indefinitely long period to have persistent control over the disease. In one patient, MMF appeared to exert a better adjuvant action than azathioprine as found by previous workers. [4] Long-term benefits of the drug on the disease are also not very apparent, as follow up after 6 months was not uniform in all the patients. A larger controlled trial is required to get the true picture of its efficacy.
| 1. |
Stanley JR. Therapy of pemphigus vulgaris. Arch Dermatol, 1999;133:76-9.
[Google Scholar]
|
| 2. |
Bystryn JC, Steinman NM. The adjuvant therapy of pemphigus: an update. Arch Dermatol, 1996;133:203-5.
[Google Scholar]
|
| 3. |
Fine JD. Management of acquired bullous skin diseases. N Eng J Med, 1995;333:1475-7.
[Google Scholar]
|
| 4. |
Enk AH, Knop J. Mycophenolate mofetil is effective in the treatment of pemphigus vulgaris. Arch Dermatol, 1998;135:54-6.
[Google Scholar]
|
| 5. |
Korman MJ. New and emerging therapies in the treatment a blistering diseases. Dermatology Clinics 2000; 18: 127-137
[Google Scholar]
|
| 6. |
Bredlich RO, Grundmann-Kollmann M, Behrens S, Kerscher M, Peter RU. Mycophenolate mofetil monotherapy for pemphigus vulgaris.Br J Dermatol. 1999;141(5):934.
[Google Scholar]
|
| 7. |
Grundman-Kollmann M, Korting HC, Behrens S, et al . Mycophenolate mofetil. A new therapeutic option in the treatment of blistering autoimmune diseases. J Am Acad Dermatol 1999; 40: 957-960
[Google Scholar]
|
| 8. |
Mimouni D, Anhalt GJ, Cummins DL, Kouba DJ, Thorne JE, Nousari HC. Treatment of pemphigus vulgaris and pemphigus foliaceus with mycophenolate mofetil. Arch Dermatol. 2003;139(6):739-42.
[Google Scholar]
|
| 9. |
Powell AM, Albert S, Al Fares S, Harman KE, Setterfield J, Bhogal B, Black MM. An evaluation of the usefulness of mycophenolate mofetil in pemphigus. Br J Dermatol. 2003;149(1):138-45.
[Google Scholar]
|
| 10. |
Nousari HC, Sragovich A, Kiyai Asadi A, et al . Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol 1999; 40: 265-8.
[Google Scholar]
|
| 11. |
Pan TD, McDonald CH. Cytotoxic agents. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy. Philadelphia: WB Saunders, 2001:180-204.
[Google Scholar]
|
Fulltext Views
5,302
PDF downloads
2,460





