Translate this page into:
N-acetylcysteine in dermatology
Correspondence Address:
Mohammad Adil
B-9 Rizvi Apartments, Medical Road, Aligarh, Uttar Pradesh
India
| How to cite this article: Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol 2018;84:652-659 |
Introduction
N-acetylcysteine is a common drug best known as an antidote for acetaminophen toxicity.[1] N-acetylcysteine has commonly been used as a mucolytic agent for many decades now. As greater details of the exact mechanism of action of n-acetylcysteine became available, it was tried in a number of diseases. It has found potential use in various medical fields ranging from neurology and psychiatry to nephrology and pulmonology. It has been used as an adjuvant therapy for the treatment of chronic obstructive pulmonary disease, contrast-induced nephropathy, Alzheimer's disease and human immunodeficiency virus (HIV).[2] These effects are largely based on the free radical scavenging property of n-acetylcysteine. This is achieved by its action as an antioxidant in times of stress due to infections, inflammation and toxins.[3] The present review aims to explore the various uses of n-acetylcysteine in the field of dermatology. This is the first such review in English literature. For the purpose of this review, we searched PubMed, Google Scholar and Scopus database using the following keywords “n-acetylcysteine in dermatology,” n-acetylcysteine in skin disease” and n-acetylcysteine in dermatology.” Review articles, original articles and case reports were included in the search.
Pharmacology
N-acetylcysteine is (2R)-2-acetamido-3-sulfanylpropanoic acid (C5H9 NO3S), a thiol compound which is an N-acetyl derivative of the endogenous amino acid, L-cysteine. It is a white crystalline powder with a characteristic sour taste and slight acetic odor and is stable at room temperature.[4]
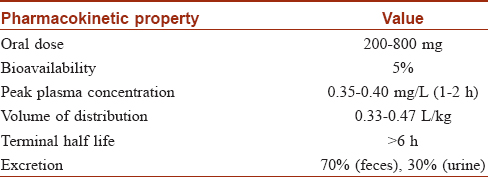
N-acetylcysteine may be used in oral, intravenous or topical routes. The usual oral dose is 200–400 mg. There is high first pass metabolism and half of the drug is bound to plasma proteins [Table - 1].[5] N-acetylcysteine rapidly gets deacetylated to form cysteine. The drug rapidly changes to its oxidized form and into metabolites which accumulate in the body.[6] Formation of disulfides on intravenous administration prolongs its half-life.[7] Topical n-acetylcysteine has a bioavailability of less than 3% and is excreted in the urine after metabolizing in the liver.[8]
Mechanism of Action
The drug mainly acts via its antioxidant effects. The mechanism of action is via one of the following:
Antioxidant action
An overproduction of reactive oxygen species leads to a state of oxidative stress which may cause damage to cellular organelles. To quench these reactive oxygen species, the cell has enzymes such as glutathione peroxidase, catalase and superoxide dismutase and sulf-hydryl compounds, of which glutathione is the most important.[9] Glutathione consists of glutamate, glycine and cysteine, the last amino acid limits its synthesis in times of stress.[9] By acting as a donor of cysteine, n-acetylcysteine causes replenishment of glutathione levels and maintains redox balance in the cells. It is important to note that n-acetylcysteine is superior to direct administration of glutathione or L-cysteine as n-acetylcysteine is less toxic, more water soluble and less susceptible to oxidation.[10] Apart from acting as a precursor to glutathione, n-acetylcysteine has also been shown to scavenge reactive oxygen species directly.[11]
Anti-inflammatory action
N-acetylcysteine has been shown to decrease the levels of IL-6 in patients on hemodialysis.[12] It has also been demonstrated that TNF-α and IL-1β is decreased in mice models treated with n-acetylcysteine.[13] N-acetylcysteine inhibits the activation of redox-sensitive nuclear factor-kappa B, which stimulates the expression of pro-inflammatory genes in times of oxidative stress, leading to release of a large amount of inflammatory cytokines.[14]
Neurotransmission modulation
Another possible mechanism by which n-acetylcysteine acts is via modulation of neurotransmitters. Cysteine dimerizes to form cystine which is transported across neurons via the cystine-glutamate antiporter and increases the inhibitory glutamate.[15] In addition, n-acetylcysteine has been demonstrated to alter dopamine levels in neurons.[16]
Antiproliferative effects
N-acetylcysteine has been shown to exert an inhibitory effect on NIH3T3 fibroblast cells of mice by reversibly blocking the early or mid G1 phase of the cell cycle. This makes n-acetylcysteine a potential drug for preventing and reversing fibrosis.[17] It also inhibits proliferation of human keratinocyte and has found utility in hyperproliferative diseases.[18]
Other actions
N-acetylcysteine also has an important role to play in vasodilatation by facilitating the production of nitric oxide.[3] It also has a role in neutrophil activation and attachment to microbes.[19]
The major mechanisms of action of n-acetylcysteine in various dermatological conditions is illustrated in [Figure - 1].
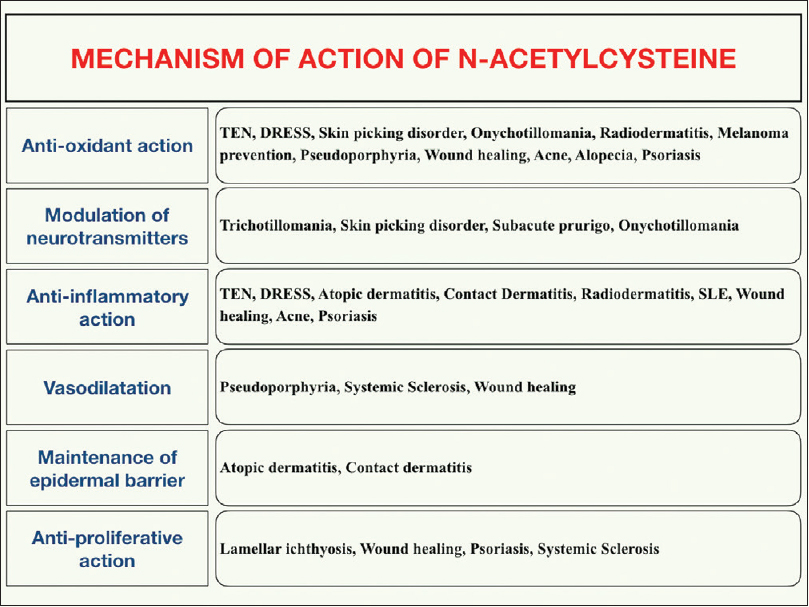 |
| Figure 1: Mechanism of action of n-acetylcysteine in various dermatological conditions |
Uses
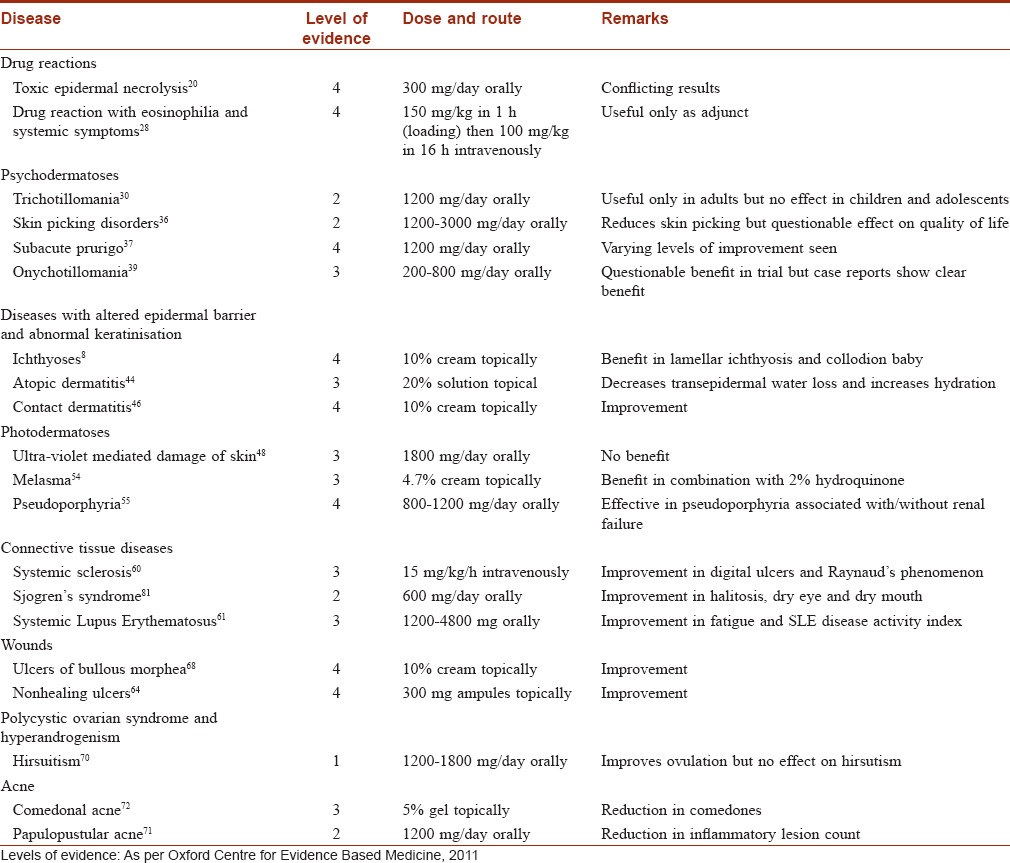
N-acetylcysteine has been used for dermatological purposes in small trials and case reports [Table - 2]. Nevertheless, it offers a safe and exciting option for the treatment of skin conditions. These have been discussed below.
Drug reactions
Toxic epidermal necrolysis
The beneficial effects of n-acetylcysteine in toxic epidermal necrolysis have been ascribed to its glutathione replenishing property, inhibition of tumor necrosis factor-α and IL-1 (the most prominent cytokines in the pathogenesis of this disease) and blockade of cutaneous lymphocyte antigen that directs the inflammatory cells to the skin.[20] Redondo et al. were the first to treat a patient of toxic epidermal necrolysis with S-adenosylmethionine, pentoxyphylline and n-acetylcysteine.[21] This was followed by another report in two patients who recovered after 300 mg/kg/day of intravenous n-acetylcysteine.[22] Similarly, patients were treated with 600 mg of intravenous n-acetylcysteine administered 8 hourly[23] and 300 mg/kg/day.[20] However, Paquet et al. found that n-acetylcysteine alone or in combination with infliximab does not alter the inflammatory cascade of toxic epidermal necrolysis and showed no improvement in overall survival.[24]
Drug reaction with eosinophilia and systemic symptoms
N-acetylcysteine acts by detoxifying reactive oxygen species produced by the offending drug, which have been implicated in the disease pathogenesis and hence the drug is effective only when given early.[25] Reports of treatment of this disease with n-acetylcysteine alone are conflicting and reviews on the management of this disease do not mention n-acetylcysteine as a treatment option.[26] Thereafter, n-acetylcysteine was used in conjunction with steroids or intravenous immunoglobulins and found to be a promising option.[27],[28]
Psychodermatological conditions
Trichotillomania
Trichotillomania is an abnormal habit and impulse control disorder characterized by a sense of tension immediately before pulling hair or when trying to resist pulling and relief of this tension after hair pulling. The mechanism of action of n-acetylcysteine in trichotillomania is believed to be the reduction of glutamate levels in the nucleus accumbens.[29] A double-blind, placebo-controlled trial was conducted on 50 patients of trichotillomania divided into two equal groups. Daily supplementation with 1200–2400 mg/day of n-acetylcysteine for a period of 12 weeks resulted in significant reductions in the severity of hair pulling compared to placebo.[30] Small case series and case reports also report benefit.[31],[32] A randomized double-blind, placebo-controlled trial on 39 children failed to show any benefit of n-acetylcysteine in trichotillomania. The authors concluded that this occurred due to the difference in the pathology of trichotillomania in children where the urge to pull hair is usually absent but hair are pulled as a habit.[33]
Skin picking disorder and prurigo
Skin picking or dermatotillomania has been classified as a distinct entity different from obsessive compulsive disorders.[34] These effects are due to the antioxidant and glutamate modulatory effects of n-acetylcysteine. N-acetylcysteine has been used in the treatment of skin picking in patients of Prader–Willi syndrome in a small case series.[35] A double-blind, randomized, placebo-controlled trial was conducted on 66 patients of skin picking disorder and n-acetylcysteine (1200–3000 mg/day orally) was found to reduce the urge or craving to pick the skin compared to placebo.[36] There was no statistically significant improvement on the quality of life in patients, which the authors attributed to the small sample size and a relatively short follow-up period of 12 weeks. Varying levels of improvement in the appearance of skin and skin picking was seen in three patients of subacute prurigo who received 1200 mg of n-acetylcysteine orally.[37] However, further studies are needed for patients with skin picking disorder before a final conclusion is drawn about its efficacy.
Onychotillomania
Nail tic disorders include nail biting or onychotillomania and onychophagia. N-acetylcysteine has been shown to reduce the symptoms of nail biting in case reports due to its effect on glutamate modulation.[38] A double-blind, placebo-controlled trial using oral n-acetylcysteine at a dose of 200–800 mg/day in children and adolescents showed that the nail length in group taking n-acetylcysteine increased significantly more than the nail length of the placebo group after 1 month of treatment, but the length was not significant after 2 months. This disparity may have been due to the continued nail growth prompting some subjects to trim their nails.[39]
Diseases with disturbed epidermal barrier/keratinization disorders
Ichthyoses
The effect is due to the anti-proliferative effects of n-acetylcysteine which diminishes the hyperkeratosis of lamellar ichthyosis and improves the skin barrier function.[8] The first article showing improvement of lamellar ichthyosis by topical n-acetylcysteine was published in 1999.[18] Another report from Turkey showed improvement in a collodion baby with 10% n-acetylcysteine oil emulsion applied twice daily over 4% urea in a split body trial.[40] The patient showed complete resolution of eclabium and ectropion by the 16th day of treatment. Similar improvements were seen in a 5-patient case series that used 10% n-acetylcysteine in 5% urea emulsion for 4 months.[8] Deffenbacher described a case with sustained benefit of n-acetylcysteine in a child.[41] An improved formula containing n-acetylcysteine has been proposed to avoid the odor emanating from the oxidation of the n-acetylcysteine cream.[42]
Atopic dermatitis
Administration of n-acetylcysteine in drinking water to flaky tail mice, the mouse model for atopic dermatitis, led to increased expression of cell adhesion molecules vital for the formation of skin barrier such as epidermal growth factor receptor, E-cadherin, occludin and sirtuin (silent mating type information regulation 2 homolog) 1.[43] A Japanese study based on these findings found that topical 20% n-acetylcysteine for 4 weeks improved the skin hydration and decreased transepidermal water loss in a majority of atopic dermatitis patients as well as healthy individuals.[44] More studies are needed to assess the role of n-acetylcysteine in atopic dermatitis.
Contact dermatitis
N-acetylcysteine is known to be protective in irritant contact dermatitis and contact hypersensitivity reactions in mouse models which is attributed to its ability to inhibit TNF-alpha by reducing nuclear factor-κB.[45] A case series reported benefit of 10% n-acetylcysteine cream applied to the back of 6 healthy volunteers who were exposed to dimethylsulfoxide.[46]
Photodermatoses
Protection from photodamage
A study on healthy adults of lighter skin color showed that n-acetylcysteine cream applied to the skin protected against photoageing by decreasing matrix metalloproteinases in skin.[47] An open-label, randomized study on humans failed to produce a statistically significant protection by N-acetylcysteine in patients of cancer on photodynamic therapy[48] despite animal studies to the contrary.[49] Topical n-acetylcysteine was reported to reduce skin reaction to radiotherapy.[50] Animal studies indicate that n-acetylcysteine may have a role as a prophylactic agent before sun exposure, and thus, may help in the prevention of malignant melanoma as it reduces oxidative stress and decreases the expression of vascular endothelial growth factor in a dose-dependent fashion.[51] It also decreases the expression of Nodal, a gene, whose expression in dysplastic nevi predicts transformation to aggressive melanoma.[52]
Melasma
N-acetylcysteine acts in melasma probably by increasing glutathione which stimulates the synthesis of pheomelanin, which is lighter in color than melanin.[53] Sulfur in n-acetylcysteine may also complex with copper to inhibit the enzyme tyrosinase.[54] N-acetylcysteine was found to reduce melasma in a double-blind, placebo-controlled study on 12 female patients who were prescribed a combination of 4.7% n-acetylcysteine and 2% hydroquinone. Mild-to-strong bleaching of the skin was observed in 90% patients.[54] No side-effects due to n-acetylcysteine were noted in any of the patients.
Pseudoporphyria
Most cases of pseudoporphyria occur in renal failure patients on hemodialysis. N-acetylcysteine is proposed to decrease oxidative stress and relieve angiopathy and hypoxia leading to this condition.[55] There are several case reports of the efficacy of this drug in hemodialysis-associated pseudoporphyria.[55],[56] Recent case reports also show benefit of n-acetylcysteine in pseudoporphyria patients who were not on hemodialysis.[57]
Collagen vascular diseases
Systemic sclerosis
N-acetylcysteine has been used in systemic sclerosis due to its effect on vasculature and inhibition of fibroblast proliferation. A one-year, double-blind, placebo-controlled trial, however, failed to show any benefit of n-acetylcysteine (500 mg orally) over placebo.[58] Another study using intravenous infusion of n-acetylcysteine (2 hour infusion of 150 mg/kg, followed by 15 mg/kg/hour for 5 days) resulted in significantly reduced digital ulcers and lower mean recovery time on cold challenge test in 22 patients of systemic sclerosis.[59] These findings were supported by another study on 50 patients of systemic sclerosis and with a mean follow-up of 3 years, which showed decreased attacks of Raynaud's phenomenon and fewer digital ulcers in the patients administered intravenous n-acetylcysteine.[60]
Systemic lupus erythematosus
Patients with systemic lupus erythematosus have low levels of glutathione in their T cells, predisposing them to pro-inflammatory cell death and necrosis. Glutathione regulates T-cell function via mammalian target of Rapamycin pathway. N-acetylcysteine blocks the mammalian target of Rapamycin pathway in T lymphocytes and improves lupus erythematosus.[61] Oral n-acetylcysteine improved fatigue and systemic lupus erythematosus disease activity index in 36 patients in a double-blind, placebo-controlled trial.[61] N-acetylcysteine has proven to be effective in systemic lupus erythematosus-induced nephritis in a case report[62] and neuropsychiatric conditions in a placebo-controlled, randomized trial.[63]
Wound healing
The effects of n-acetylcysteine on wound healing are related to its action as an antioxidant, support of nitric oxide system, stimulation of cell proliferation, migration and collagenous expression of matrix metalloproteinases.[64] Experimental studies done mostly in animals have reported that n-acetylcysteine is effective in various types of wounds such as burns,[65] incisional wounds[66] and wounds after radiotherapy.[67] Recently, there was a report of healing of non-healing pressure ulcers in two patients with n-acetylcysteine topically applied to the wounds.[64] Ulcers caused by bullous morphea were treated in a patient with n-acetylcysteine and topical wound care.[68]
Polycystic ovarian syndrome and hyperandrogenism
N-acetylcysteine is proposed to benefit in polycystic ovarian syndrome by lowering insulin secretion and restoring the deranged hormonal profile in affected patients. In an open-label trial, 100 patients were randomized to receive metformin or 1800 mg daily of n-acetylcysteine for 24 weeks. N-acetylcysteine produced significant reduction in free testosterone and hirsutism scores at 6 months after treatment and the results were comparable to the effect produced by metformin.[69] Though n-acetylcysteine has been used in patients of polycystic ovarian syndrome and resulting hyperandrogenism in several clinical trials, a recent meta-analysis of eight clinical trials has shown that acne and hirsutism do not improve significantly when compared with placebo.[70]
Acne
While n-acetylcysteine helps improve inflammatory lesions of acne by quenching reactive oxygen species, inhibition of leukotrienes and prostaglandins, stabilization of membranes and inhibiting lipid peroxidation,[71] its effect on comedones is less well defined and could be due to decreased sebum production or inhibition of Pityrosporum ovale, a potential comedogenic organism that has been demonstrated in comedones.[72] A randomized, single-blind trial from Iraq showed that the number of inflammatory lesions decreased significantly in 14 patients who received 1200 mg/day of n-acetylcysteine compared to placebo.[71] Another double-blind, placebo-controlled trial found that the test group comprising 65 patients who received 5% n-acetylcysteine gel for 8 weeks had significantly reduced the number of comedones compared to patients in the control group (34 patients) who received only placebo.[72]
Other potential uses
The role of n-acetylcysteine in androgenetic alopecia is proposed as it has been shown to reduce the free radicals and decrease the senescence of balding human dermal papillae obtained from patients of androgenetic alopecia.[73] Animal studies show that n-acetylcysteine may also help in preventing chemotherapy-induced alopecia.[74] Reactive oxygen species play a role in the tumor necrosis factor-α induced inflammatory pathways in human keratinocytes. n-acetylcysteine, being an antioxidant, was found to play a role in the suppression of this pathway.[75] This finding paves the way for further studies on the role of n-acetylcysteine in psoriasis. While in-vitro studies supported the idea that n-acetylcysteine may be useful in the treatment of methemoglobinemia, a crossover, randomized trial on human volunteers with induced methemoglobinemia demonstrated that intravenous n-acetylcysteine offers no reduction in methemoglobin concentration in blood.[76]
Adverse Effects
N-acetylcysteine is usually safe when administered orally in the dose of 2400 mg/day or lower.[3] The drug has a strong, disagreeable flavor and it needs to be taken with fruit juice or soft drink for this reason.[10] Side-effects at this dose are mild and include nausea, vomiting, diarrhea, flushing, pain in the epigastric region, constipation and skin rash. At higher dosage, urticaria, chills, skin rash, headache, tinnitus and fever may be seen.[10] The frequency of side-effects with oral administration of the drug is at least as much as that of intravenous administration.[77] Intravenous administration of the drug may lead to an anaphylactoid reaction, which may present as urticarial rash, pruritus, angioedema, bronchospasm and hypotension. Females and those with atopic diathesis are more prone to develop this type of reaction, which is supposedly produced by non-immunological mechanism under the influence of histamine.[78] N-acetylcysteine may also destabilize the disulfide bonds in clotting factors and modestly prolong the International Normalized Ratio, though this may not be clinically significant.[79] Topical use of n-acetylcysteine is generally safe and may be associated with mild side-effects such as burning, erythema and pruritus.[41]
N-acetylcysteine should be avoided in patients taking nitroglycerin or related medications as this may cause hypotension by vasodilatory effects and by reversing the tolerance to nitrates.[80] Use of n-acetylcysteine in pregnancy is not indicated unless absolutely necessary due to the dearth of studies in pregnant women. Excretion of the drug in breast milk or its crossing the placental barrier in pregnancy is controversial and animal studies have shown doubtful embryotoxicity.[5]
Conclusion
Though an old drug, the potential of n-acetylcysteine has probably not been fully recognized in dermatology. It is a relatively safe drug, and can be used intravenously, orally and topically. The effects of n-acetylcysteine are largely based on its antioxidant and anti-inflammatory properties and its modulation of neurotransmitters. However, the studies evaluating its efficacy are few and small and have shown only modest benefit. More controlled human studies are needed to confirm its efficacy in various skin diseases. Furthermore, correct dosage regimens need to be established for the optimal utilization of this drug in various dermatological conditions.[81]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev 2006;2:CD003328.
[Google Scholar]
|
| 2. |
Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin Biol Ther 2008;8:1955-62.
[Google Scholar]
|
| 3. |
Millea PJ. N-acetylcysteine: Multiple clinical applications. Am Fam Physician 2009;80:265-9.
[Google Scholar]
|
| 4. |
N-acetyl-L-cysteine. National Center for Biotechnology Information. Pubchem Compound Database; CID 12035. Available from: https://www.pubchem.ncbi.nlm.nih.gov/compound/12035. [Last accessed on 2017 Dec 20].
[Google Scholar]
|
| 5. |
Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav 2014;4:108-22.
[Google Scholar]
|
| 6. |
Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet 1991;20:123-34.
[Google Scholar]
|
| 7. |
Sarker KP, Abeyama K, Nishi J, Nakata M, Tokioka T, Nakajima T, et al. Inhibition of thrombin-induced neuronal cell death by recombinant thrombomodulin and E5510, a synthetic thrombin receptor signaling inhibitor. Thromb Haemost 1999;82:1071-7.
[Google Scholar]
|
| 8. |
Bassotti A, Moreno S, Criado E. Successful treatment with topical N-acetylcysteine in urea in five children with congenital lamellar ichthyosis. Pediatr Dermatol 2011;28:451-5.
[Google Scholar]
|
| 9. |
Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr 2005;2:38-44.
[Google Scholar]
|
| 10. |
Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-acetylcysteine – A safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 2007;7:355-9.
[Google Scholar]
|
| 11. |
Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 2011;36:78-86.
[Google Scholar]
|
| 12. |
Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, et al. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: A placebo-controlled study. Perit Dial Int 2010;30:336-42.
[Google Scholar]
|
| 13. |
Chen G, Shi J, Hu Z, Hang C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: A potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm 2008;2008:716458.
[Google Scholar]
|
| 14. |
Gloire G, Piette J. Redox regulation of nuclear post-translational modifications during NF-kappaB activation. Antioxid Redox Signal 2009;11:2209-22.
[Google Scholar]
|
| 15. |
Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 2005;25:6389-93.
[Google Scholar]
|
| 16. |
Gere-Pászti E, Jakus J. The effect of N-acetylcysteine on amphetamine-mediated dopamine release in rat brain striatal slices by ion-pair reversed-phase high performance liquid chromatography. Biomed Chromatogr 2009;23:658-64.
[Google Scholar]
|
| 17. |
Sekharam M, Trotti A, Cunnick JM, Wu J. Suppression of fibroblast cell cycle progression in G1 phase by N-acetylcysteine. Toxicol Appl Pharmacol 1998;149:210-6.
[Google Scholar]
|
| 18. |
Redondo P, Bauzá A. Topical N-acetylcysteine for lamellar ichthyosis. Lancet 1999;354:1880.
[Google Scholar]
|
| 19. |
Allegra L, Dal Sasso M, Bovio C, Massoni C, Fonti E, Braga PC. Human neutrophil oxidative bursts and their in vitro modulation by different N-acetylcysteine concentrations. Arzneimittelforschung 2002;52:669-76.
[Google Scholar]
|
| 20. |
Vélez A, Moreno JC. Toxic epidermal necrolysis treated with N-acetylcysteine. J Am Acad Dermatol 2002;46:469-70.
[Google Scholar]
|
| 21. |
Redondo P, Ruiz de Erenchun F, Iglesias ME, Monedero P, Quintanilla E. Toxic epidermal necrolysis. Treatment with pentoxifylline. Br J Dermatol 1994;130:688-9.
[Google Scholar]
|
| 22. |
Saavedra C, Cárdenas P, Castellanos H, Contreras K, Castro JR. Cephazolin-induced toxic epidermal necrolysis treated with intravenous immunoglobulin and N-acetylcysteine. Case Reports Immunol 2012;2012:931528.
[Google Scholar]
|
| 23. |
Yavuz H, Emiroglu M. Toxic epidermal necrolysis treated with N-acetylcysteine. Pediatr Int 2014;56:e52-4.
[Google Scholar]
|
| 24. |
Paquet P, Jennes S, Rousseau AF, Libon F, Delvenne P, Piérard GE. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. A proof-of-concept study. Burns 2014;40:1707-12.
[Google Scholar]
|
| 25. |
Moling O, Tappeiner L, Piccin A, Pagani E, Rossi P, Rimenti G, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir – A hypothesis. Med Sci Monit 2012;18:CS57-62.
[Google Scholar]
|
| 26. |
Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): A clinical update and review of current thinking. Clin Exp Dermatol 2011;36:6-11.
[Google Scholar]
|
| 27. |
Jose J, Klein R. Successful treatment of sulfasalazine-induced DRESS syndrome with corticosteroids and N-acetylcysteine. Pharmacotherapy 2011;31:1043.
[Google Scholar]
|
| 28. |
Cumbo-Nacheli G, Weinberger J, Alkhalil M, Thati N, Baptist AP. Anticonvulsant hypersensitivity syndrome: Is there a role for immunomodulation? Epilepsia 2008;49:2108-12.
[Google Scholar]
|
| 29. |
Odlaug BL, Grant JE. N-acetyl cysteine in the treatment of grooming disorders. J Clin Psychopharmacol 2007;27:227-9.
[Google Scholar]
|
| 30. |
Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: A double-blind, placebo-controlled study. Arch Gen Psychiatry 2009;66:756-63.
[Google Scholar]
|
| 31. |
Rodrigues-Barata AR, Tosti A, Rodríguez-Pichardo A, Camacho-Martínez F. N-acetylcysteine in the treatment of trichotillomania. Int J Trichology 2012;4:176-8.
[Google Scholar]
|
| 32. |
Taylor M, Bhagwandas K. N-acetylcysteine in trichotillomania: A panacea for compulsive skin disorders? Br J Dermatol 2014;171:1253-5.
[Google Scholar]
|
| 33. |
Bloch MH, Panza KE, Grant JE, Pittenger C, Leckman JF. N-acetylcysteine in the treatment of pediatric trichotillomania: A randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Adolesc Psychiatry 2013;52:231-40.
[Google Scholar]
|
| 34. |
Lochner C, Roos A, Stein DJ. Excoriation (skin-picking) disorder: A systematic review of treatment options. Neuropsychiatr Dis Treat 2017;13:1867-72.
[Google Scholar]
|
| 35. |
Miller JL, Angulo M. An open-label pilot study of N-acetylcysteine for skin-picking in Prader-Willi syndrome. Am J Med Genet A 2014;164A: 421-4.
[Google Scholar]
|
| 36. |
Grant JE, Chamberlain SR, Redden SA, Leppink EW, Odlaug BL, Kim SW. N-acetylcysteine in the treatment of excoriation disorder: A randomized clinical trial. JAMA Psychiatry 2016;73:490-6.
[Google Scholar]
|
| 37. |
Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol 2015;72 Suppl 1:AB117.
[Google Scholar]
|
| 38. |
Magid M, Mennella C, Kuhn H, Stamu-O'Brien C, Kroumpouzos G. Onychophagia and onychotillomania can be effectively managed. J Am Acad Dermatol 2017;77:e143-4.
[Google Scholar]
|
| 39. |
Ghanizadeh A, Derakhshan N, Berk M. N-acetylcysteine versus placebo for treating nail biting, a double blind randomized placebo controlled clinical trial. Antiinflamm Antiallergy Agents Med Chem 2013;12:223-8.
[Google Scholar]
|
| 40. |
Sarici SU, Sahin M, Yurdakök M. Topical N-acetylcysteine treatment in neonatal ichthyosis. Turk J Pediatr 2003;45:245-7.
[Google Scholar]
|
| 41. |
Deffenbacher B. Successful experimental treatment of congenital ichthyosis in an infant. BMJ Case Rep 2013;2013. pii: bcr2013008688.
[Google Scholar]
|
| 42. |
Davila-Seijo P, Flórez A, Davila-Pousa C, No N, Ferreira C, De la Torre C. Topical N-acetylcysteine for the treatment of lamellar ichthyosis: An improved formula. Pediatr Dermatol 2014;31:395-7.
[Google Scholar]
|
| 43. |
Nakai K, Yoneda K, Hosokawa Y, Moriue T, Presland RB, Fallon PG, et al. Reduced expression of epidermal growth factor receptor, E-cadherin, and occludin in the skin of flaky tail mice is due to filaggrin and loricrin deficiencies. Am J Pathol 2012;181:969-77.
[Google Scholar]
|
| 44. |
Nakai K, Yoneda K, Murakami Y, Koura A, Maeda R, Tamai A, et al. Effects of topical N-acetylcysteine on skin hydration/Transepidermal water loss in healthy volunteers and atopic dermatitis patients. Ann Dermatol 2015;27:450-1.
[Google Scholar]
|
| 45. |
Senaldi G, Pointaire P, Piguet PF, Grau GE. Protective effect of N-acetylcysteine in hapten-induced irritant and contact hypersensitivity reactions. J Invest Dermatol 1994;102:934-7.
[Google Scholar]
|
| 46. |
Pasche-Koo F, Arechalde A, Arrighi JF, Hauser C. Effect of N-acetylcysteine, an inhibitor of tumor necrosis factor, on irritant contact dermatitis in the human. Curr Probl Dermatol 1995;23:198-206.
[Google Scholar]
|
| 47. |
Kang S, Chung JH, Lee JH, Fisher GJ, Wan YS, Duell EA, et al. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol 2003;120:835-41.
[Google Scholar]
|
| 48. |
Baas P, van Mansom I, van Tinteren H, Stewart FA, van Zandwijk N. Effect of N-acetylcysteïne on photofrin-induced skin photosensitivity in patients. Lasers Surg Med 1995;16:359-67.
[Google Scholar]
|
| 49. |
Baas P, Oppelaar H, van der Valk MA, van Zandwijk N, Stewart FA. Partial protection of photodynamic-induced skin reactions in mice by N-acetylcysteine: A preclinical study. Photochem Photobiol 1994;59:448-54.
[Google Scholar]
|
| 50. |
Kim JA, Baker DG, Hahn SS, Goodchild NT, Constable WC. Topical use of N-acetylcysteine for reduction of skin reaction to radiation therapy. Semin Oncol 1983;10:86-92.
[Google Scholar]
|
| 51. |
Redondo P, Bandrés E, Solano T, Okroujnov I, García-Foncillas J. Vascular endothelial growth factor (VEGF) and melanoma. N-acetylcysteine downregulates VEGF production in vitro. Cytokine 2000;12:374-8.
[Google Scholar]
|
| 52. |
Margaryan NV, Gilgur A, Seftor EA, Purnell C, Arva NC, Gosain AK, et al. Melanocytes affect nodal expression and signaling in melanoma cells: A lesson from pediatric large congenital melanocytic nevi. Int J Mol Sci 2016;17:418.
[Google Scholar]
|
| 53. |
Sehgal VN, Verma P, Srivastava G, Aggarwal AK, Verma S. Melasma: Treatment strategy. J Cosmet Laser Ther 2011;13:265-79.
[Google Scholar]
|
| 54. |
Njoo MD, Menke HE, Pavel W, Westerhof W. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol 1997;9:86-7.
[Google Scholar]
|
| 55. |
Vadoud-Seyedi J, de Dobbeleer G, Simonart T. Treatment of haemodialysis-associated pseudoporphyria with N-acetylcysteine: Report of two cases. Br J Dermatol 2000;142:580-1.
[Google Scholar]
|
| 56. |
Cooke NS, McKenna K. A case of haemodialysis-associated pseudoporphyria successfully treated with oral N-acetylcysteine. Clin Exp Dermatol 2007;32:64-6.
[Google Scholar]
|
| 57. |
Katoulis AC, Ferra D, Toumbis E, Papadavid E, Kanelleas A, Panayiotides I, et al. Pseudoporphyria associated with nonhemodialyzed renal insufficiency, successfully treated with oral N-acetylcysteine. Case Rep Dermatol Med 2013;2013:271873.
[Google Scholar]
|
| 58. |
Furst DE, Clements PJ, Harris R, Ross M, Levy J, Paulus HE. Measurement of clinical change in progressive systemic sclerosis: A 1 year double-blind placebo-controlled trial of N-acetylcysteine. Ann Rheum Dis 1979;38:356-61.
[Google Scholar]
|
| 59. |
Sambo P, Amico D, Giacomelli R, Matucci-Cerinic M, Salsano F, Valentini G, et al. Intravenous N-acetylcysteine for treatment of Raynaud's phenomenon secondary to systemic sclerosis: A pilot study. J Rheumatol 2001;28:2257-62.
[Google Scholar]
|
| 60. |
Rosato E, Borghese F, Pisarri S, Salsano F. The treatment with N-acetylcysteine of Raynaud's phenomenon and ischemic ulcers therapy in sclerodermic patients: A prospective observational study of 50 patients. Clin Rheumatol 2009;28:1379-84.
[Google Scholar]
|
| 61. |
Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2012;64:2937-46.
[Google Scholar]
|
| 62. |
Tewthanom K, Janwitayanujit S, Totemchockcyakarn K, Panomvana Na Ayudhya D. The effect of high dose of N-acetylcysteine in lupus nephritis: A case report and literature review. J Clin Pharm Ther 2010;35:483-5.
[Google Scholar]
|
| 63. |
Garcia RJ, Francis L, Dawood M, Lai ZW, Faraone SV, Perl A. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum 2013;65:1313-8.
[Google Scholar]
|
| 64. |
Ozkaya H, Bahat G, Tufan A, Dogan H, Bilicen Z, Karan MA. Successful treatment of non-healing pressure ulcers with topical n-acetyl cysteine. J Wound Care 2015;24:606, 608-11.
[Google Scholar]
|
| 65. |
Deniz M, Borman H, Seyhan T, Haberal M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns 2013;39:320-5.
[Google Scholar]
|
| 66. |
Demir EO, Cakmak GK, Bakkal H, Turkcu UO, Kandemir N, Demir AS, et al. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J Invest Surg 2011;24:151-8.
[Google Scholar]
|
| 67. |
Aktunc E, Ozacmak VH, Ozacmak HS, Barut F, Buyukates M, Kandemir O, et al. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol 2010;35:902-9.
[Google Scholar]
|
| 68. |
Rosato E, Veneziano ML, Di Mario A, Molinaro I, Pisarri S, Salsano F. Ulcers caused by bullous morphea: Successful therapy with N-acetylcysteine and topical wound care. Int J Immunopathol Pharmacol 2013;26:259-62.
[Google Scholar]
|
| 69. |
Oner G, Muderris II. Clinical, endocrine and metabolic effects of metformin vs. N-acetyl-cysteine in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2011;159:127-31.
[Google Scholar]
|
| 70. |
Thakker D, Raval A, Patel I, Walia R. N-acetylcysteine for polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Obstet Gynecol Int 2015;2015:817849.
[Google Scholar]
|
| 71. |
Sahib AS, Al-Anbari HH, Salih M, Abdullah F. Effect of oral antioxidants on lesion counts associated with oxidative stress and inflammation in patients with papulopustular acne. J Clin Exp Dermatol Res 2012;3:5.
[Google Scholar]
|
| 72. |
Montes LF, Wilborn WH, Montes CM. Topical acne treatment with acetylcysteine: Clinical and experimental effects. Skinmed 2012;10:348-51.
[Google Scholar]
|
| 73. |
Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol 2015;135:1244-52.
[Google Scholar]
|
| 74. |
D'Agostini F, Bagnasco M, Giunciuglio D, Albini A, De Flora S. Inhibition by oral N-acetylcysteine of doxorubicin-induced clastogenicity and alopecia, and prevention of primary tumors and lung micrometastases in mice. Int J Oncol 1998;13:217-24.
[Google Scholar]
|
| 75. |
Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J Invest Dermatol 2008;128:2606-14.
[Google Scholar]
|
| 76. |
Tanen DA, LoVecchio F, Curry SC. Failure of intravenous N-acetylcysteine to reduce methemoglobin produced by sodium nitrite in human volunteers: A randomized controlled trial. Ann Emerg Med 2000;35:369-73.
[Google Scholar]
|
| 77. |
Perry HE, Shannon MW. Efficacy of oral versus intravenous N-acetylcysteine in acetaminophen overdose: Results of an open-label, clinical trial. J Pediatr 1998;132:149-52.
[Google Scholar]
|
| 78. |
Pakravan N, Waring WS, Sharma S, Ludlam C, Megson I, Bateman DN. Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin Toxicol (Phila) 2008;46:697-702.
[Google Scholar]
|
| 79. |
Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila) 2009;47:81-8.
[Google Scholar]
|
| 80. |
Ardissino D, Merlini PA, Savonitto S, Demicheli G, Zanini P, Bertocchi F, et al. Effect of transdermal nitroglycerin or N-acetylcysteine, or both, in the long-term treatment of unstable angina pectoris. J Am Coll Cardiol 1997;29:941-7.
[Google Scholar]
|
| 81. |
Walters MT, Rubin CE, Keightley SJ, Ward CD, Cawley MI. A double-blind, cross-over, study of oral N-acetylcysteine in Sjögren's syndrome. Scand J Rheumatol Suppl 1986;61:253-8.
[Google Scholar]
|
Fulltext Views
52,211
PDF downloads
4,894





