Translate this page into:
Nail changes in autoimmune blistering disorders: A case-control study
Correspondence Address:
Manjunath Mala Shenoy
Department of Dermatology, Venereology and Leprosy, Yenepoya Medical College, Yenepoya University, Deralakatte, Mangalore - 575 018, Karnataka
India
| How to cite this article: Gopal V, Shenoy MM, Bejai V, Nargis T. Nail changes in autoimmune blistering disorders: A case-control study. Indian J Dermatol Venereol Leprol 2018;84:373 |
Abstract
Background: Pemphigus and pemphigoid disorders produce blistering cutaneous lesions. Earlier case reports state that nail involvement is uncommon in these autoimmune blistering disorders.
Aims and Objectives: To study nail changes in autoimmune blistering disorders.
Methods: A case-control study was conducted where 40 cases and 40 controls were evaluated for nail changes.
Results: Nail changes were seen in 72.5% of cases and 17.5% of controls. The most common nail findings were paronychia and onychorrhexis.
Limitations: Small sample size; short study duration; nail biopsy could not be done.
Conclusion: Our findings indicate that the inflammatory nature of the blistering cutaneous disease is often reflected conspicuously in the nails.
Introduction
Autoimmune blistering disorders including pemphigus and the pemphigoid group are characterised by autoantibodies against desmosomal or basement membrane zone proteins, and manifest primarily with cutaneous and oral lesions. The incidence of nail changes in these disorders, which has been thought to be rare, has been found to be high in a few recent studies.[1] Nail involvement may occur as the target antigens are expressed in the proximal nail fold, nail matrix, and hyponychium. It may correlate with the severity of mucocutaneous symptoms and disease duration.[1] Nail manifestations can also be a result of the extension of adjacent bullae to the nail.[2] Appropriate treatment improves the cutaneous as well as nail lesions. In this study, we evaluated the incidence of nail changes in autoimmune blistering disorders and compared it with that in an age- and sex-matched control group.
Methods
Forty consecutive cases of autoimmune blistering disorders attending the dermatology outpatient department of Yenepoya Medical College, Mangalore, Karnataka, from October 2016 to December 2016, were included in this study after obtaining ethical clearance. All old and new cases confirmed by histopathology and direct immunofluorescence were included. Forty healthy controls of corresponding age and sex were selected at random and taken as a comparative group. (Age and sex matched controls were selected at random). Complete dermatological examination was performed with special attention to nail changes by all the study authors. Photographic documentation of mucocutaneous lesions and nails were done after obtaining patients' consent. Nail changes in both cases and controls were interpreted separately by two experienced dermatologists, Dr Manjunath M.S. and Dr Vishal B. Collected data were analyzed by both descriptive and inferential statistical methods. Data frequency was measured using percentages, means and standard deviations. Significance was measured using a Chi-square test, P < 0.05 being considered significant. Analysis was performed using SPSS software version 16 (SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago, IL 60606-6412).
Results
Twenty-nine (72.5%) out of the 40 cases and 7 (17.5%) out of the 40 controls had nail changes The mean age of patients was 44.5 ± 12.9 years. The male-to-female ratio was 1:2.1 in both the groups. The frequency of nail changes in each blistering disorder is shown in [Figure - 1] and the frequencies of various nail changes are as in [Table - 1].
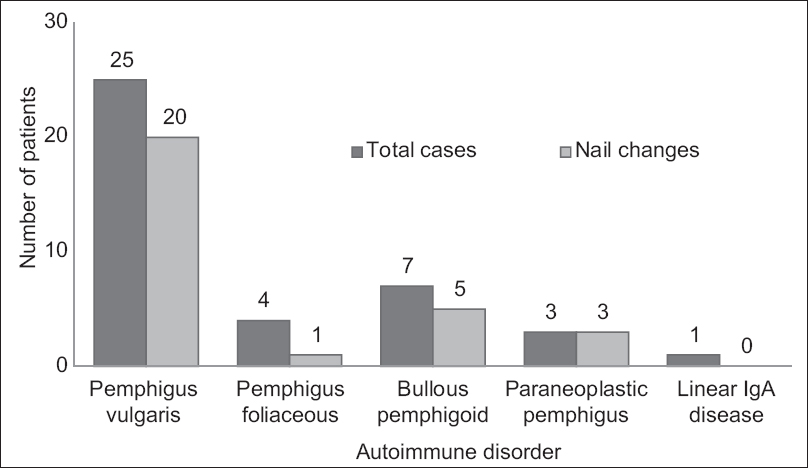 |
| Figure 1: Frequency of nail changes in autoimmune bullous disorders |
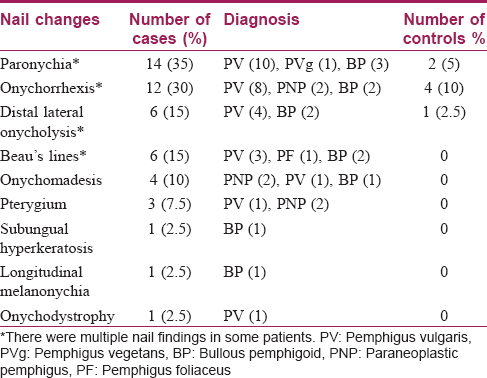
The presence of paronychia, onychorrhexis, distal lateral onycholysis, Beau's lines, onychomadesis and pterygium in the case group was found to be statistically significant (P< 0.05) compared to controls.
The duration of disease at presentation varied widely (1 to 24 months) with an average of 5.8 months. Average disease duration was 2.9 months in those without nail changes, and 7 months in those with nail changes. The average duration between disease onset and nail changes was 4.2 months for onychorrhexis, 4.2 months for paronychia, 2.5 months for distal lateral onycholysis, and 3 months for Beau's lines. Twenty-two (55%) patients presented with an acute flare of their disease, and nail involvement was present in all these cases.
Based on the percentage of body surface area involved, the autoimmune blistering disorder was graded as mild (≤20% body surface area) or severe (>20% of body surface area). Twenty-one out of the 23 cases with severe disease had nail changes. This association between severity of disease and nail changes was found statistically significant (P = 0.0021). Further, using the Pemphigus Disease Area Index scoring system, mucosal involvement was also categorised as mild (score <15), moderate (score = 15–45), or severe (score >45). Out of the 28 patients with mucosal involvement, 18(64.3%) had mild involvement, 7 (25%) moderate, and 3 (10.7%) had severe involvement. Nail involvement was seen in 13 patients out of those with mild mucosal involvement and in all patients with moderate or severe mucosal involvement. However, these findings were not found to be statistically significant (P = 0.082).
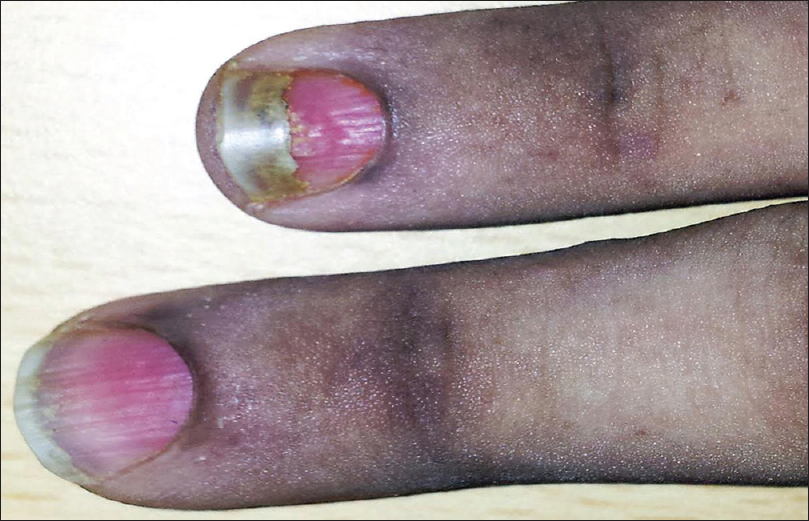
Out of the 25 patients with pemphigus vulgaris, 20 (80%) had nail changes including paronychia (40%) [Figure - 2], onychorrhexis (32%), onychomycosis (16%), Beau's lines (12%), and onychomadesis (4%). Eight had painful acute paronychia around fingernails, along with extensive blistering over the upper extremities [Figure - 2]. Another two had chronic paronychia from long before disease onset, attributed to regular prolonged contact with water. In both these cases, nail- fold swelling increased after the onset of pemphigus [Figure - 3]. Two controls also had chronic paronychia attributable to prolonged contact with water. We did not observe paronychia to be a sign heralding exacerbation prior to onset of disease, nor did we elicit such a history from patients in our study.
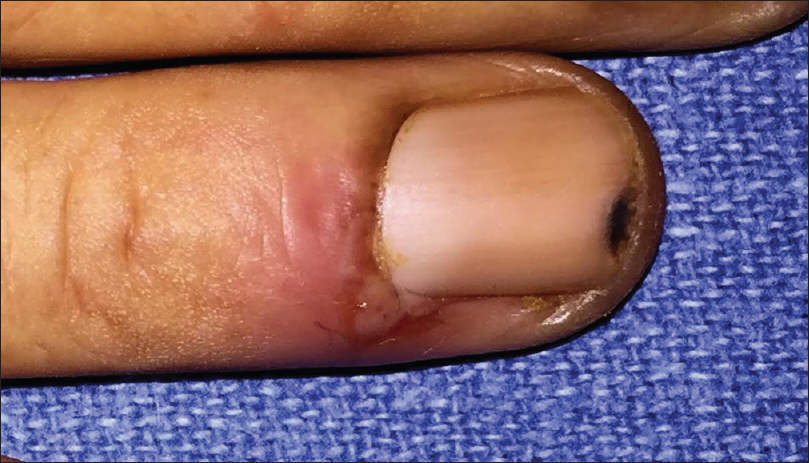 |
| Figure 2: Acute paronychia involving the right ring finger in a patient with pemphigus vulgaris |
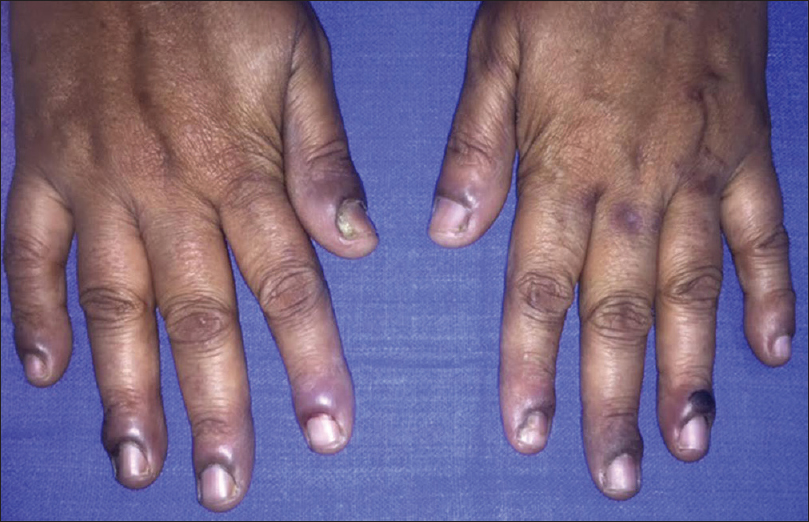 |
| Figure 3: Acute exacerbation of chronic paronychia in patient with pemphigus vulgaris with periungual bulla |
Nail changes were seen in 5 out of 7 cases of bullous pemphigoid and in all 3 cases of paraneoplastic pemphigus (pterygium, onychomaedesis, and onychorrhexis). On the other hand, out of 3 cases of pemphigus foliaceus, only 1 with extensive blisters had Beau's lines [Figure - 4].
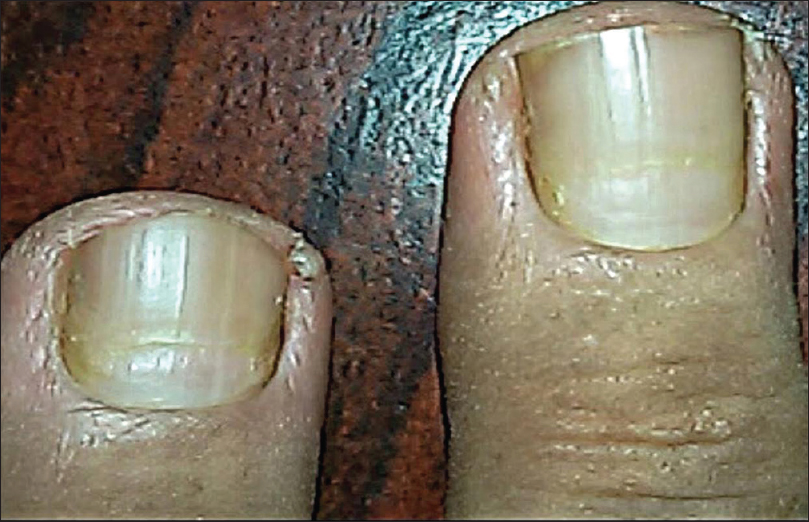 |
| Figure 4: Beau's lines on the middle and ring fingernails in patient with pemphigus foliaceus |
Paronychia was the most common finding overall, seen in 14 (35%) cases. It was also the most frequent (12/22, 54.5%) nail change seen after disease exacerbation. Six patients who had had repeated exacerbations were found to have multiple Beau's lines.
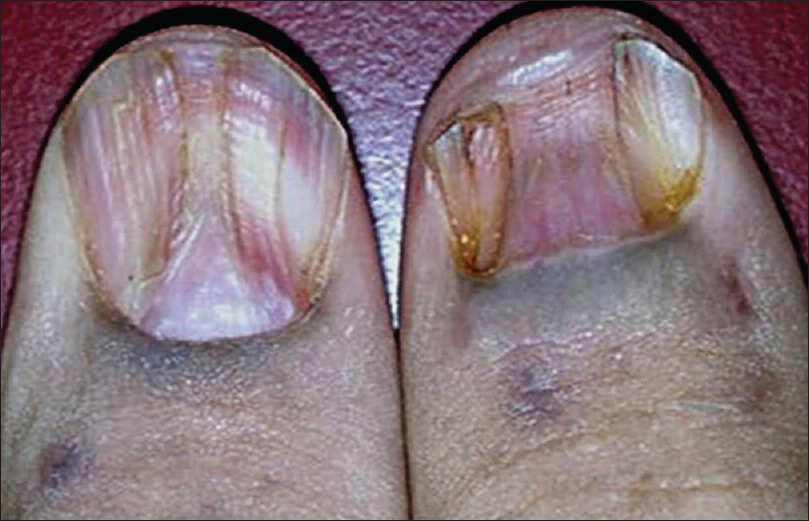
Onychorrhexis was noted in 12 (30%) patients [Figure - 5]. Out of the 12, four had only mild ridging without any discomfort, present from before the onset of disease. These 4 patients (2 each with pemphigus vulgaris and bullous pemphigoid) were between 55 and 80 years of age.
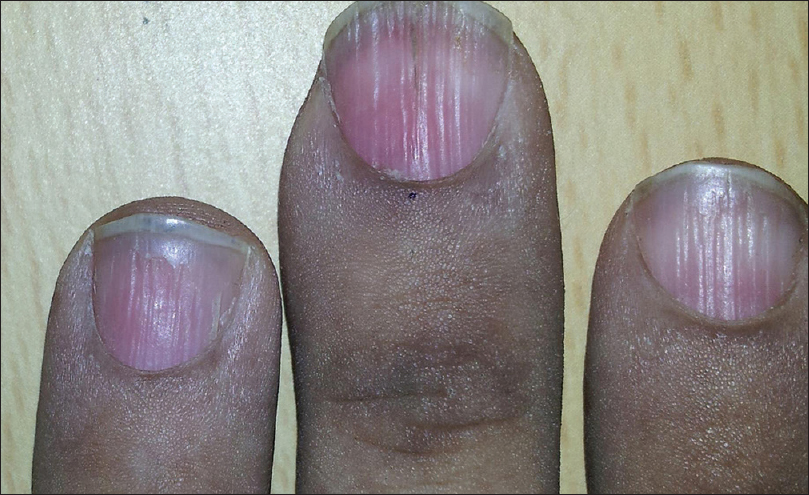 |
| Figure 5: Onychorrhexis involving the fingernails in a paraneoplastic pemphigus patient |
Onychorrhexis was mainly noted in the older age group and was seen above the age of 45 years in 11 out of 12 cases, Only one younger (37-year-old male) patient with paraneoplastic pemphigus had onychorrhexis. The association of onychorrhexis with age was found to be significant in the case group (P< 0.001) as well as in the control group (P = 0.017).
Six patients had periungual bullae and all of them had pemphigus vulgaris [Figure - 3] and [Figure - 6]. Paronychia was seen in the involved and adjacent nails in 5 of these cases. Fingernails were more commonly affected than toenails. Tzanck smears from the periungual bullae showed acantholytic cells. The association of periungual bullae with paronychia was significant (P = 0.042). Three of these cases also had distal lateral subungual onychomycosis (confirmed by potassium hydroxide mounts), and 1 had onychomadesis [Figure - 7].
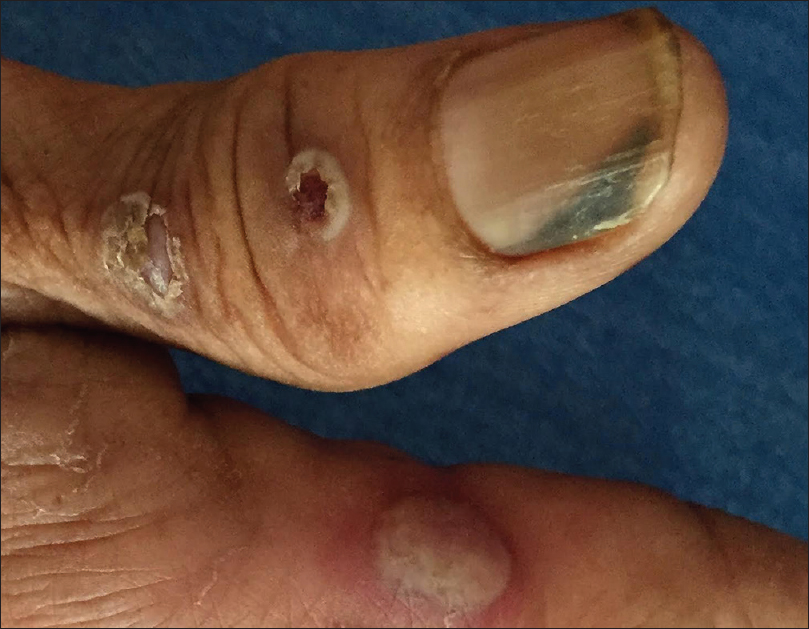 |
| Figure 6: Distal lateral onycholysis in a patient with pemphigus vulgaris |
 |
| Figure 7: Onychomadesis over the right ring finger in a pemphigus vegetans patient |
Six patients who were on immunosuppressive therapy had distal lateral onycholysis suggestive of onychomycosis [Figure - 6]. Pterygium formation was seen in 2 cases of paraneoplastic pemphigus [Figure - 8] and 1 case of severe pemphigus vulgaris. Other nail manifestations such as subungual hyperkeratosis (2.5%, 1/40), onychodystrophy (2.5%, 1/40), pits (2.5%, 1/40), and longitudinal melanonychia (2.5%, 1/40) were also seen.
 |
| Figure 8: Pterygium of thumb nails in a patient with paraneoplastic pemphigus |
Discussion
Sinclair et al. showed that staining for epidermal hemidesmosomal and basement membrane zone antigens do not differ in the nail. These antigens are also expressed in the nail fold, nail matrix, and hyponychium.[3] Autoimmune blistering disorders are relatively uncommon dermatoses and very few studies have looked at nail changes in them. We found only one review by Tosti et al. on nail changes in these disorders.[1]
Pemphigus and pemphigoid disorders have been extensively studied in terms of etiology, clinical manifestations, and therapeutic protocols. Though earlier literature described nail involvement in these disorders as uncommon,[4] some recent studies indicate otherwise. Nail changes are common in patients with long-standing disease because of accumulated inflammatory effects,[1] though in our study, nail involvement occurred from 1 month to 24 months after disease onset.
Nail fold changes have been reported in pemphigus.[2],[4],[5],[6],[7],[8] In pemphigus vulgaris, paronychia is regarded as a sign heralding exacerbation.[9] It was seen in 11 out of 25 cases in our study, including 1 case of pemphigus vegetans. In all, it developed either with the initial presentation or at the time of exacerbations. However, we did not observe paronychia prior to the onset of mucocutaneous lesions.[8] Paronychia occurs as a result of acantholysis of lateral nail fold epidermis and usually resolves with immunosuppressant therapy.[10] Staphylococcus aureus and Candida albicans have been isolated in acute paronychia due to pemphigus vulgaris.[11]
Onychorrhexis was the second most common finding in our study. This is the longitudinal ridging of the nail plate, often associated with nail thinning, and indicates defective keratinization of the proximal nail matrix. Mild ridging is common in old age which might explain its significant persistence in the older age group even after treatment, as well as its occurrence in controls of similar age. However, it was seen in both our cases of paraneoplastic pemphigus and in 6 cases of pemphigus vulgaris after disease onset, wherein it may have occurred because of nail matrix damage.[12]
There is a higher prevalence of onychomycosis in patients with autoimmune diseases. Six of our patients who were on immunosuppressive therapy developed distal lateral onycholysis suggestive of onychomycosis. Tuchinda et al. reported that 24% patients with vesiculobullous autoimmune disorders, most of them on immunosuppressive therapy, had onychomycosis. Apart from immunosuppression, a humid environment can also favor this.[13] The duration of onychomycosis has also been found to be longer in these patients. The most common causative organism isolated was Candida species.[14]
Beau's lines occur due to the temporary arrest of proximal nail matrix activity leading to the formation of transverse depressions. Their distance from the proximal nail fold reflects the time from disease onset and multiple lines correspond to recurrent exacerbations.[12] This latter feature was seen in 6 of our cases.
Onychomaedesisis the proximal detachment of the nail plate from the nail bed and occurs as a result of a severe underlying inflammatory process that causes complete arrest of nail matrix activity.[12] Autoimmune blistering disorders can inhibit normal nail plate growth and development. Alternatively, blister formation beneath the nail plate can also cause this change.[15] In our study, it was seen in 3 cases, 2 with paraneoplastic pemphigus and the other with pemphigus vulgaris.
Pterygium formation, seen in 3 of our patients, occurs as a result of focal destruction of the nail matrix and scarring, and is usually preceded by an inflammatory destructive process. Common causes include trauma, lichen planus and its variants, and graft-versus-host disease.[16]
Some other reported nail findings such as subungual hemorrhage, trachyonychia, and nail rippling were not seen in our study.[2],[4],[8],[17]
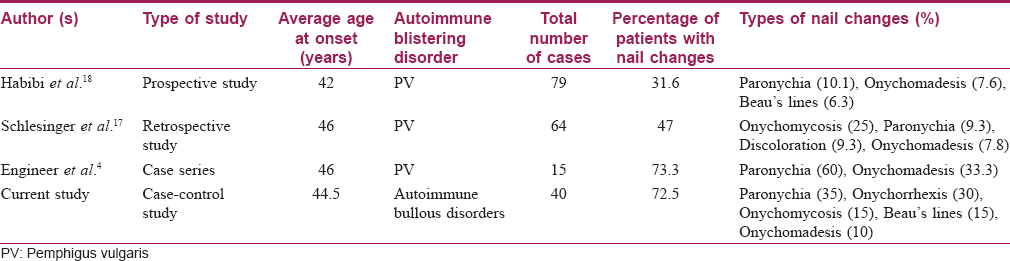
Nail involvement in pemphigus occurs due to bullous lesions in the nail bed/nail matrix, or acantholysis of the lateral nail fold as a part of the disease process.[1],[4],[11] Paronychia and onychomadesis are the most common nail findings reported in pemphigus vulgaris. Habibi et al. reported that 31.6% of pemphigus vulgaris patients had nail changes, paronychia and onychomadesis being the most common.[18] Engineer et al. reviewed the reports of nail involvement in 15 patients of pemphigus vulgaris; they too noted that the most frequent alterations were paronychia (60%) and onychomadesis (33%).[4] Similarly Cahali et al. described 5 patients with this disorder presenting with nail involvement; all 5 had paronychia and 1 had Beau's lines. Only fingernails were involved.[19] Schlesinger et al. found nail involvement in 30 (47%) out of 64 pemphigus vulgaris patients. Of these, onycomycosis was seen in 53.3% (34/64).[17]
Bullous pemphigoid mostly affects the nail folds, although nail bed and matrix can also be involved. Involvement is probably due to an immunologic reaction involving the bullous pemphigoid antigen. The location of blistering in the nail apparatus determines the appearance of nail changes.[1] Nail changes in bullous pemphigoid are considered rare; onychomadesis, nail scarring, paronychia, and pterygium have been reported.[1],[20],[21],[22],[23] However, we noticed a higher frequency of nail changes in this condition.
There are not many reports of nail changes in paraneoplastic pemphigus, though all 3 cases in our study had nail changes. The cutaneous manifestations of this disease simulate those of lichen planus, graft-versus-host disease, erythema multiforme, pemphigus and pemphigoid, disorders which can produce nail changes seen in our paraneoplastic pemphigus patients.[24]
[Table - 2] summarizes the nail findings in autoimmune blisteting disorders as described by other investigators.
Our final analysis is that the nail changes caused primarily by the damaging effects of autoimmune blistering disorders on the nail apparatus are paronychia, onychorrhexis, Beau's lines, and onychomadesis. Secondary nail changes are onychomycosis predisposed to by immunosuppression, and pterygium formation as a sequel of nail matrix scarring.
The response of nail changes to therapy was not analyzed in this study due to its short duration. However, we observed that paronychia which had occurred after disease onset resolved with immunosuppressive therapy. Onychomycosis was treated with topical antifungal agents over a period of months. Other changes such as pterygia were irreversible.
Limitations
A limitation of this study was its small sample size. Nail matrix biopsies would have helped differentiate nail changes due to the blistering disorders from changes caused by other preexisting conditions, but most patients did not consent to this invasive procedure. Onychoscopy was done only in a few cases, and the findings therein have not been analyzed in this study.
Conclusion
Nail examination is warranted in every case of autoimmune blistering disorder; the inflammatory nature of the blistering disease is often conspicuously reflected in the nails. Nails may herald exacerbation of the underlying disease. Nail changes also seem to be more common in those with longer disease duration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Tosti A, André M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin 2011;29:511-3, xi.
[Google Scholar]
|
| 2. |
Kim BS, Song KY, Youn JI, Chung JH. Paronychia - A manifestation of pemphigus vulgaris. Clin Exp Dermatol 1996;21:315-7.
[Google Scholar]
|
| 3. |
Sinclair RD, Wojnarowska F, Leigh IM, Dawber RP. The basement membrane zone of the nail. Br J Dermatol 1994;131:499-505.
[Google Scholar]
|
| 4. |
Engineer L, Norton LA, Ahmed AR. Nail involvement in pemphigus vulgaris. J Am Acad Dermatol 2000;43:529-35.
[Google Scholar]
|
| 5. |
Rivera Diaz R, Alonso Llamazares J, Rodriguez Peralto JL, Sebastian Vanaclocha F, Iglesias Diez L. Nail involvement in pemphigus vulgaris. Int J Dermatol 1996;35:581-2.
[Google Scholar]
|
| 6. |
Berker DD, Dalziel K, Dawber RP, Wojnarowska F. Pemphigus associated with nail dystrophy. Br J Dermatol 1993;129:461-4.
[Google Scholar]
|
| 7. |
Dhawan SS, Zaias N, Pena J. The nail fold in pemphigus vulgaris. Arch Dermatol 1990;126:1374-5.
[Google Scholar]
|
| 8. |
Lauber J, Turk K. Beau's lines and pemphigus vulgaris. Int J Dermatol 1990;29:309.
[Google Scholar]
|
| 9. |
Akiyama C, Sou K, Furuya T, Saitoh A, Yasaka N, Ohtake N, et al. Paronychia: A sign heralding an exacerbation of pemphigus vulgaris. J Am Acad Dermatol 1993;29:494-6.
[Google Scholar]
|
| 10. |
Serratos BD, Rashid RM. Nail disease in pemphigus vulgaris. Dermatol Online J 2009;15:2.
[Google Scholar]
|
| 11. |
Lee HE, Wong WR, Lee MC, Hong HS. Acute paronychia heralding the exacerbation of pemphigus vulgaris. Int J Clin Pract 2004;58:1174-6.
[Google Scholar]
|
| 12. |
Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorrizo JL, Schaffer JV, editors. Dermatology. 3rd ed. China: Elsevier; 2012. p. 1129-47.
[Google Scholar]
|
| 13. |
Tuchinda P, Boonchai W, Prukpaisarn P, Maungprasat C, Suthipinittharm P. Prevalence of onychomycosis in patients with autoimmune diseases. J Med Assoc Thai 2006;89:1249-52.
[Google Scholar]
|
| 14. |
Boonchai W, Kulthanan K, Maungprasat C, Suthipinittham P. Clinical characteristics and mycology of onychomycosis in autoimmune patients. J Med Assoc Thai 2003;86:995-1000.
[Google Scholar]
|
| 15. |
Patsatsi A, Sotiriou E, Devliotou-Panagiotidou D, Sotiriadis D. Pemphigus vulgaris affecting 19 nails. Clin Exp Dermatol 2009;34:202-5.
[Google Scholar]
|
| 16. |
de Berker DA, Baran R. Nail disorders. In: Burns T, Brethnach S, Cox N, Griffiths C, editors. Rooks Textbook of Dermatology. 8th ed. West Sussex: Willey Blackwell; 2010. p. 1-54, 65.
[Google Scholar]
|
| 17. |
Schlesinger N, Katz M, Ingber A. Nail involvement in pemphigus vulgaris. Br J Dermatol 2002;146:836-9.
[Google Scholar]
|
| 18. |
Habibi M, Mortazavi H, Shadianloo S, Balighi K, Ghodsi SZ, Daneshpazhooh M, et al. Nail changes in pemphigus vulgaris. Int J Dermatol 2008;47:1141-4.
[Google Scholar]
|
| 19. |
Cahali JB, Kakuda EY, Santi CG, Maruta CW. Nail manifestations in pemphigus vulgaris. Rev Hosp Clin Fac Med Sao Paulo 2002;57:229-34.
[Google Scholar]
|
| 20. |
Benmously-Mlika R, Hammami-Ghorbel H, Mokhtar I. Onychomadesis during bullous pemphigoid. J Am Acad Dermatol 2013;69:e306-7.
[Google Scholar]
|
| 21. |
Gualco F, Cozzani E, Parodi A. Bullous pemphigoid with nail loss. Int J Dermatol 2005;44:967-8.
[Google Scholar]
|
| 22. |
Namba Y, Koizumi H, Kumakiri M, Hashimoto T, Muramatsu T, Ohkawara A. Bullous pemphigoid with permanent loss of the nails. Acta Derm Venereol 1999;79:480-1.
[Google Scholar]
|
| 23. |
Tomita M, Tanei R, Hamada Y, Fujimura T, Katsuoka K. A case of localized pemphigoid with loss of toenails. Dermatology 2002;204:155.
[Google Scholar]
|
| 24. |
Wieczorek M, Czernik A. Paraneoplastic pemphigus: A short review. Clin Cosmet Investig Dermatol 2016;9:291-5.
[Google Scholar]
|
Fulltext Views
6,872
PDF downloads
4,367





