Translate this page into:
Narrow-band ultraviolet B comb as an effective home-based phototherapy device for limited or localized non-segmental vitiligo: A pilot, open-label, single-arm clinical study
2 Department of Community and Family Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
Correspondence Address:
Sujay Khandpur
Room No 4071, 4th Floor Teaching Block, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029
India
| How to cite this article: Khandpur S, Bhatia R, Bhadoria AS. Narrow-band ultraviolet B comb as an effective home-based phototherapy device for limited or localized non-segmental vitiligo: A pilot, open-label, single-arm clinical study. Indian J Dermatol Venereol Leprol 2020;86:298-301 |
Sir,
Narrow-band (NB) ultraviolet B (UVB) is an established modality in vitiligo. NB-UVB therapy delivered by whole-body chambers is available only in specialized centers, requires regular hospital visits, is expensive, and inconvenient to patients traveling long distances for therapy. These short-comings can be addressed by handheld home-based phototherapy devices. Two pilot trials have shown that this is a feasible, safe, and effective treatment for vitiligo.[1],[2] However, sufficient data on its efficacy, optimum dosing regimen, and impact on quality of life (QoL) is lacking. We undertook a preliminary study to assess the utility of a handheld NB-UVB comb in limited non-segmental vitiligo.
This was a prospective, open-label, single-arm clinical trial conducted in the Department of Dermatology and Venereology at All India Institute of Medical Sciences, New Delhi, after obtaining institute ethical clearance and registering the trial.
Adult patients (>18 years of age) with nonsegmental localized vitiligo (≤2% body surface area or ≤10 patches) were included in the study. Patients with rapidly spreading lesions, acro-facial vitiligo or with predominant leucotrichia, and pregnant/lactating women were excluded. All subjects provided a written informed consent.
All patients were given detailed instructions regarding the use of the NB-UVB comb (V-Care Meditech Pvt. Ltd., Bangalore, India). The protocol was based on our earlier published study on dosimetry and calibration of the NB-UVB combs.[3] After switching on the lamp for 45 s, patients exposed the patches to the light. The first patch to be exposed was the representative patch, the exposure time being 70 s, followed by sequential exposure of the next four patches for 80, 80, 90, and 100 s, respectively. The comb was switched off for 5 min due to significant decay in irradiance and then the next five patches were exposed sequentially for similar time. The irradiance of the NB-UVB comb was measured using a UV meter (Herbert Waldmann GmbH and Co. KG Villingen-Schwenningen, Germany). The fluence to be delivered to each patch was kept constant at 500 mJ/cm[2], and the exposure time was calculated. The patients repeated the therapy on alternate days for 6 months. They were followed up biweekly for the first 1 month and then monthly until 6 months.
The assessment parameters included percentage repigmentation of the representative patch using graphical method, percentage global repigmentation of vitiligo using Lund and Browder score, Investigator Global Assessment (IGA) of repigmentation (done by two independent dermatologists, on a 7-point Likert scale), Patient Global Assessment (PGA) on a 0–10 VAS scale, and change in dermatology life quality index, using Tjioe et al.'s questionnaire with scale ranging from –28 to +28.
Statistical analysis was done using SPSS version 22.0. Descriptive statistics included presentation of categorical variable as a frequency or proportion. Continuous variables were presented as either mean with standard deviation or median with interquartile range. Appropriate statistical tests were used. A P value < 0.05 was considered significant.
Eleven cases were eligible for recruitment, of which one patient dropped out after the first visit due to personal issues. Ten patients with mean age 30.4 ± 10 (range 21–54) years were included [Table - 1].

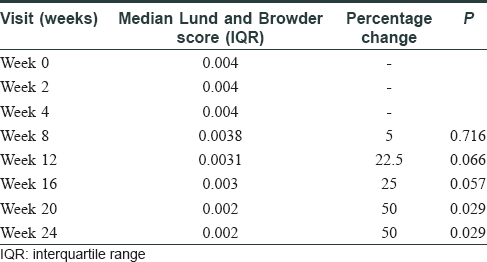
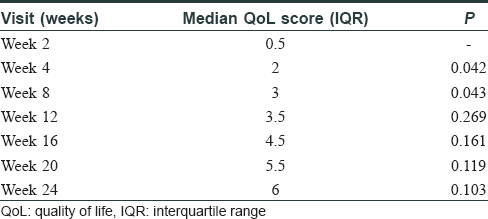
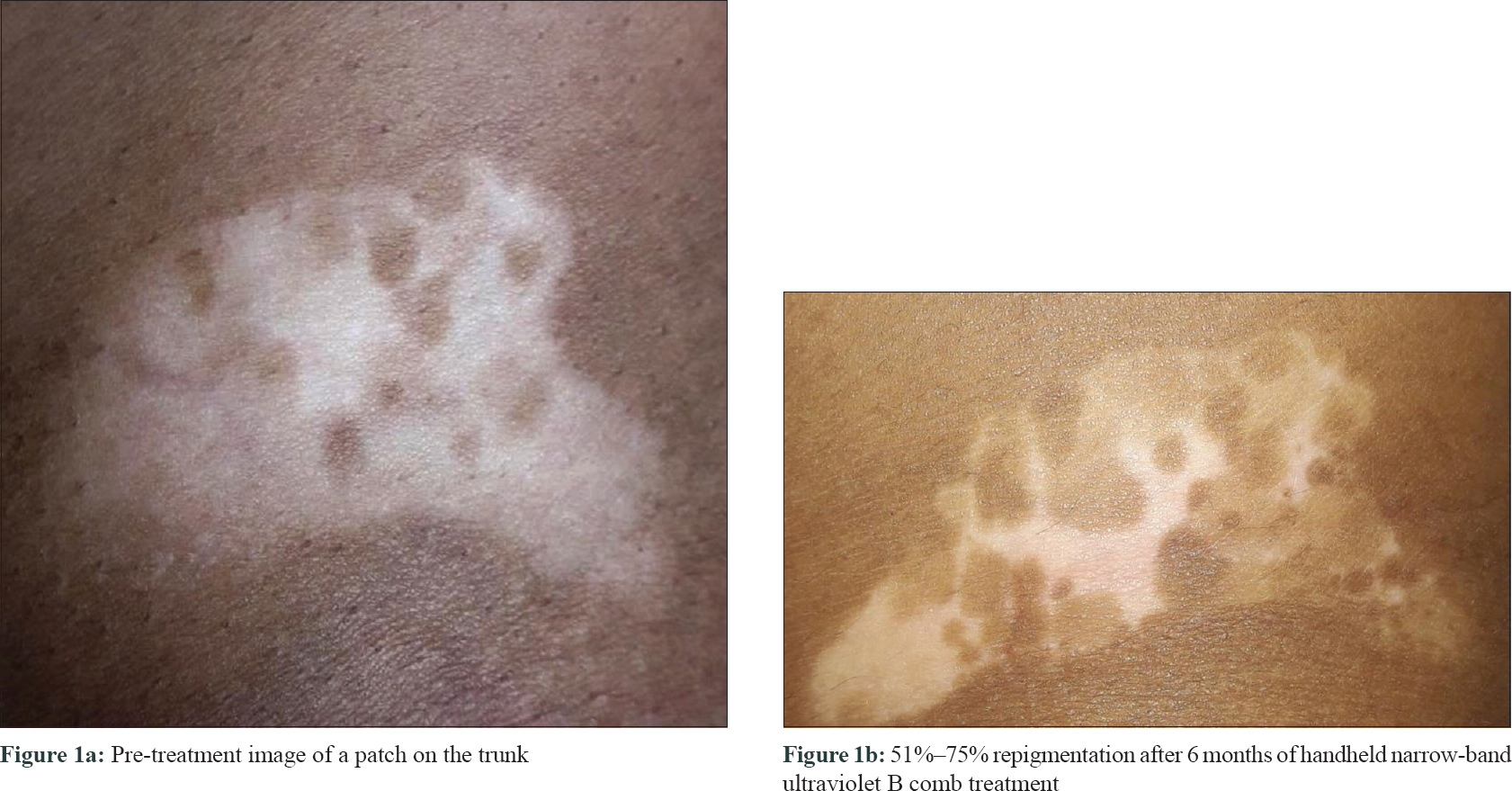
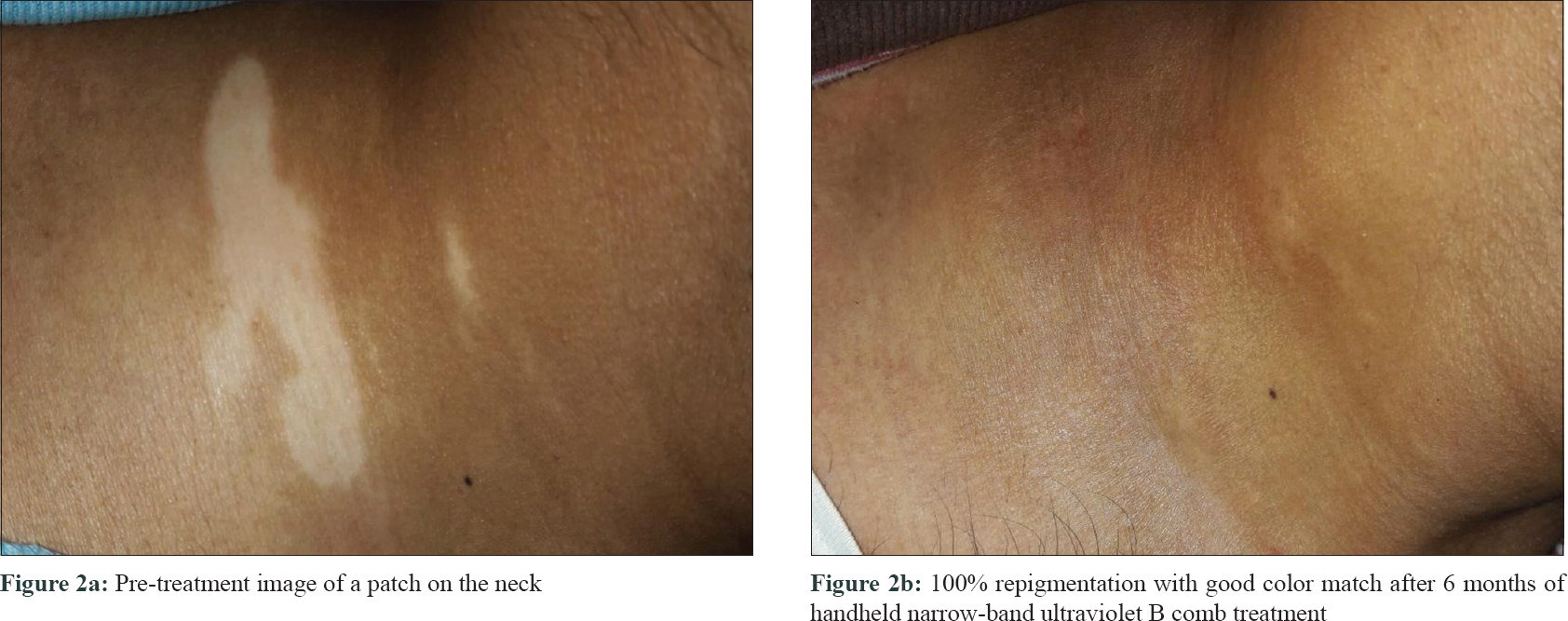
The median-affected area of the representative patch decreased progressively at every visit: from 4.46 cm2 at baseline to 3.02 cm2 at 3 months (P = 0.03) and to 1.88 cm[2] at 6 months (P = 0.009). There was a median reduction in size by 58%. The median overall body surface area involved by vitiligo decreased by 50% at 6 months (P = 0.029) [Table - 2]. PGA showed improvement by a median of 3 points (P = 0.006) at 3 months and 5.75 points (P = 0.001) at 6 months. The QoL scores reduced by a median of 6 points at 6 months (P = 0.103) [Table - 3]. At 6 months, six patients (60%) had 50%–75% improvement, 2 (20%) had 25%–50%, and one (10%) had> 90% improvement, while one patient did not perceive any change [Figure - 1], [Figure - 2], [Figure - 3].
 |
| Figure 1: |
 |
| Figure 2: |
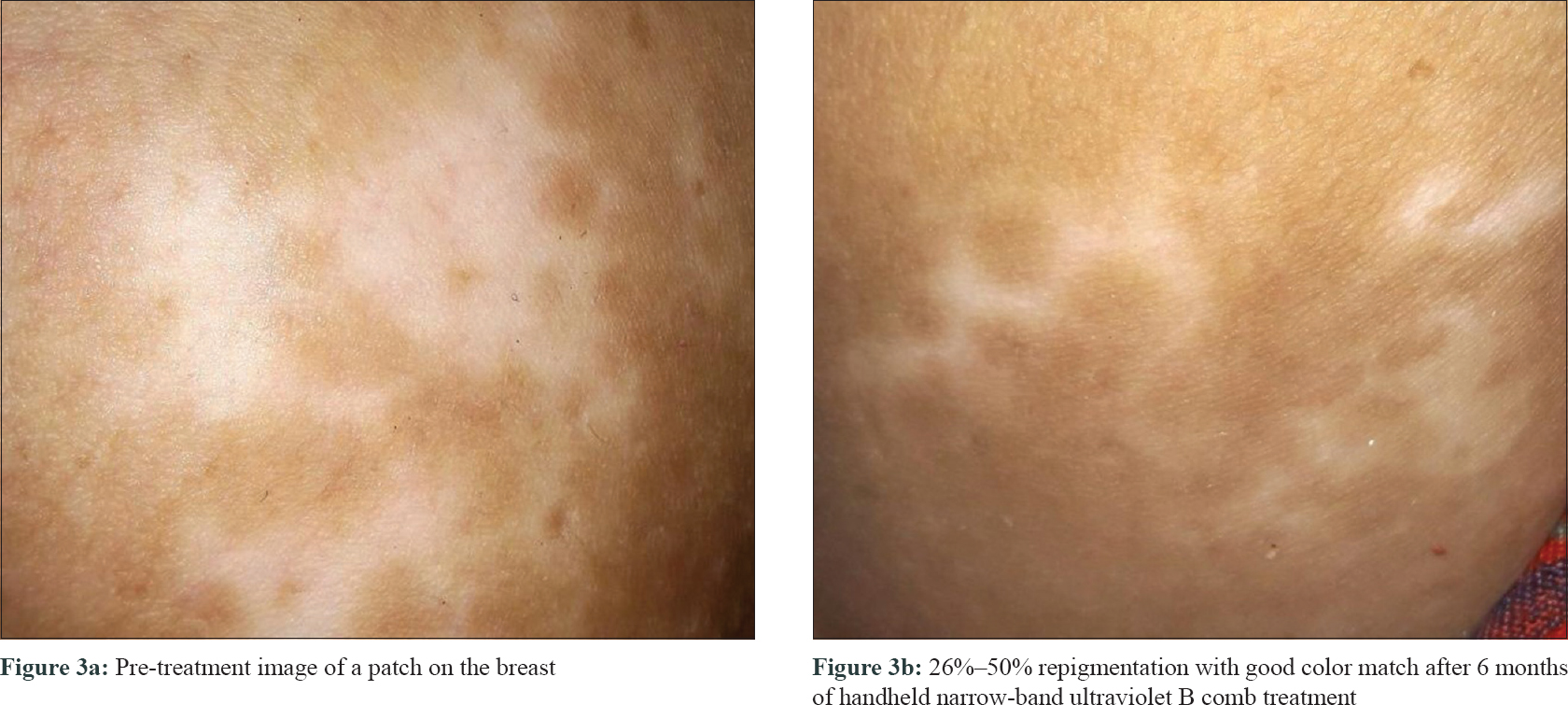 |
| Figure 3: |
Treatment was well-tolerated by all cases, except one who developed an episode of grade 3 erythema.
Phototherapy is an established safe and effective therapy for vitiligo. The European Dermatology Forum Consensus guidelines recommend total body NB-UVB as a first-line therapy for active, widespread disease.[4] Handheld NB-UVB units are portable and lightweight devices.
The HI-Light trial for vitiligo conducted in 2012 in Nottingham using two different handheld NB-UVB devices, Dermfix 1000 NB-UVB and Waldmann NB-UVB 109, found that after 4 months of treatment, high-grade repigmentation (75%–100%) was noted in 12% participants with 75% of lesions in the active group showing some repigmentation compared with 39% in the placebo group.[1] A study by Shan et al. involving 93 vitiligo patients treated with handheld NB-UVB device showed some repigmentation (>25%) in 88% cases, with excellent repigmentation (>75%) in 42% cases.[2] In our study, 90% of cases showed some repigmentation at 6 months. It was high grade (>90%) in 10%, moderate in 60%, and low grade in 20% cases. Color match was rated as good to excellent in one-third of the repigmented lesions in the former study, whereas in our study there was complete color match in 44% of cases. About 90% of our cases showed a 51% improvement in QoL. One incident of erythema grade 3 was reported in the Nottingham trial.[1] We also observed one case of grade 3 erythema, attributed to mistaken daily dosing instead of alternate day dosing by the patient.
An important lacuna in prior studies is the lack of information on the dosing regimen of their devices. We found a decay in irradiance of about 20%–30% at 3 months, requiring increase in exposure time of around 30–40 s. Moreover with our device, the irradiance reduced to very low levels after 5 min of continuous use, so the equipment had to be switched off for 5 min for the irradiance to again build up and then re-expose the remaining patches.[3] In the Nottingham trial, the exposure time per sitting was 15–30 s depending on the skin phototype, with an updosing of 3–6 s until a maximum of 3–6 min of exposure.[1] Shan et al. administered doses independent of skin type, starting with 0.3 J/cm2 with increments of 0.1 J/cm[2] per se ssion until a dose causing pink erythema was reached.[2]
Handheld NB-UVB device is a potentially useful, safe, and easy to administer home-based phototherapy option in localized or limited non-segmental vitiligo. Given that it is in the initial stages of use in this disease, a periodic calibration-based treatment schedule is of utmost importance for optimum dosing.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The handheld NB-UVB combs were purchased from departmental funds of the Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H, et al. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-light trial: Home intervention of light therapy). Trials 2014;15:51.
[Google Scholar]
|
| 2. |
Shan X, Wang C, Tian H, Yang B, Zhang F. Narrow-band ultraviolet B home phototherapy in vitiligo. Indian J Dermatol Venereol Leprol 2014;80:336-8.
[Google Scholar]
|
| 3. |
Khandpur S, Bhatia R. Handheld narrow band ultraviolet B comb as home phototherapy device for localised vitiligo: Dosimetry and calibration. Indian J Dermatol Venereol Leprol 2018;84:78-80.
[Google Scholar]
|
| 4. |
Taieb A, Alomar A, Böhm M, Dell'anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013;168:5-19.
[Google Scholar]
|
Fulltext Views
4,873
PDF downloads
2,685





