Translate this page into:
Nasal extranodal NK/T-cell lymphoma presenting as a perforating palatal ulcer: A diagnostic challenge
Correspondence Address:
Vidya Kharkar
Department of Dermatology, Seth GS Medical College and KEM Hospital, Parel, Mumbai 12
India
| How to cite this article: Patel V, Mahajan S, Kharkar V, Khopkar U. Nasal extranodal NK/T-cell lymphoma presenting as a perforating palatal ulcer: A diagnostic challenge. Indian J Dermatol Venereol Leprol 2006;72:218-221 |
Abstract
A 40-year-old man presented with chronic nasal stuffiness and bloodstained discharge of 3 years' duration, along with a non-healing palatal ulcer since 2 months. Examination revealed a perforation in the midline on the hard palate and a superficial ulcer on the soft palate. Histopathology and immunohistochemistry suggested a diagnosis of extranodal nasal/nasal-type T-cell lymphoma. The patient was started on multiagent chemotherapy in the form of cyclophosphamide, doxorubicin, vincristine and prednisolone but succumbed after two cycles. Only one case of nasal T cell lymphoma presenting as nasal septal perforation, oronasal fistula and a concomitant palatal ulcer has been described. We report this case of a perforating palatal ulcer as a rare presentation of nasal lymphoma.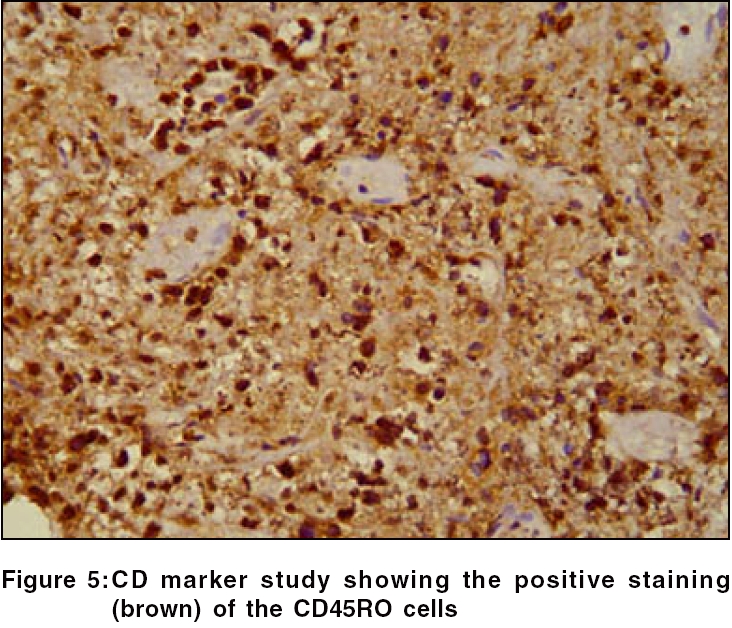 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Introduction
Nasal NK/T-cell lymphomas are aggressive, locally destructive, midfacial necrotizing lesions. Most of them were initially diagnosed as ′lethal midline granuloma′,[1],[2],[3] a term that is slowly being phased out. The stumbling block in the early diagnosis and management of these lymphomas is the nonspecificity of the symptoms, which usually predate the ulceration by years. Thus we need to be aware of the seemingly innocuous presentation of this lymphoma. To the best of our knowledge, this is the only case of nasal NK/T-cell lymphoma presenting solely as a palatal ulcer.
Case report
A 40-year-old male presented with complaints of chronic nasal stuffiness with bloodstained discharge since 3 years. There was a recent history of development of palatal ulceration, nasal twang and regurgitation of food.
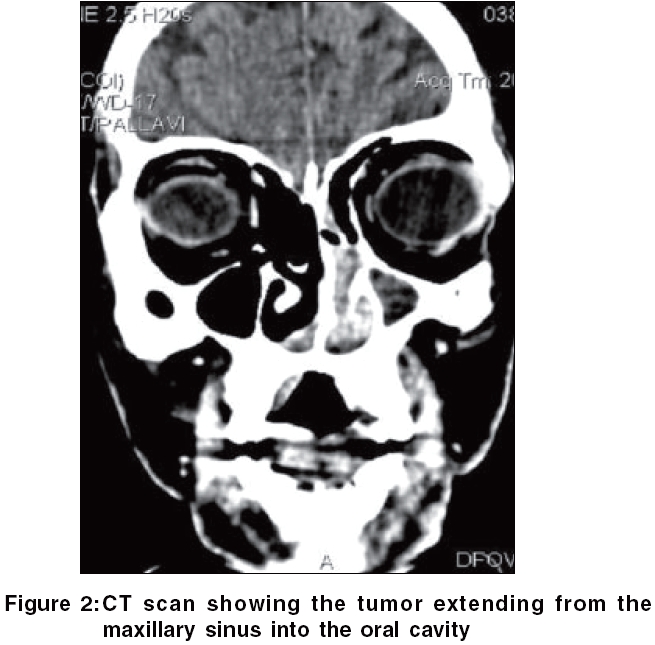
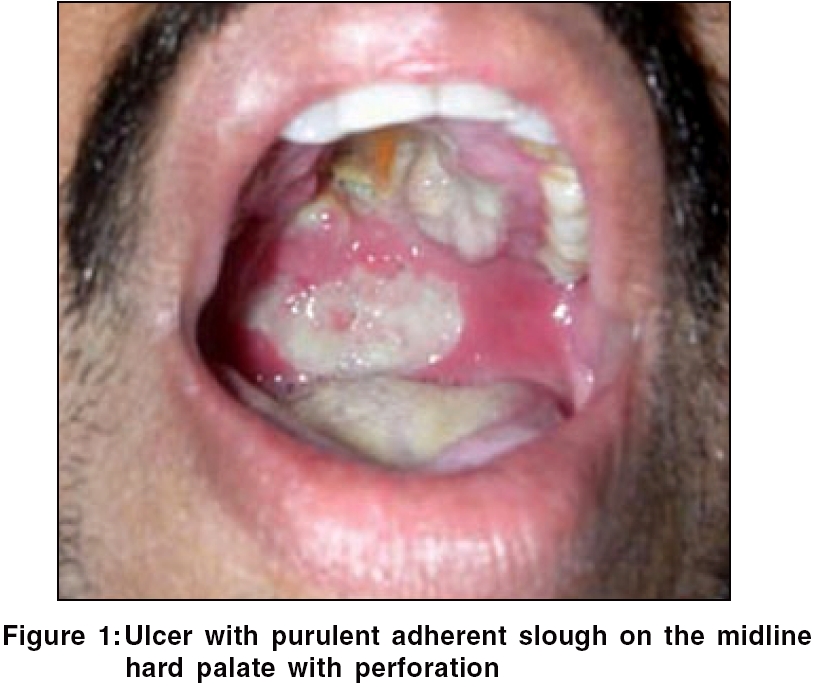
General examination was unremarkable. Oral examination revealed a tender perforating ulcer with irregular margins and undermined edges situated in the midline on the hard palate, with a similar but superficial ulcer situated on the soft palate. Profuse purulent adherent slough was present on the floor of the ulcer [Figure - 1]. An ulcero-granulomatous growth was seen in the left anterior nares. The growth progressively increased to involve the upper gingiva with fullness of the left cheek within 10 days. Meanwhile, the palatal ulcer showed an obvious perforation in the midline.
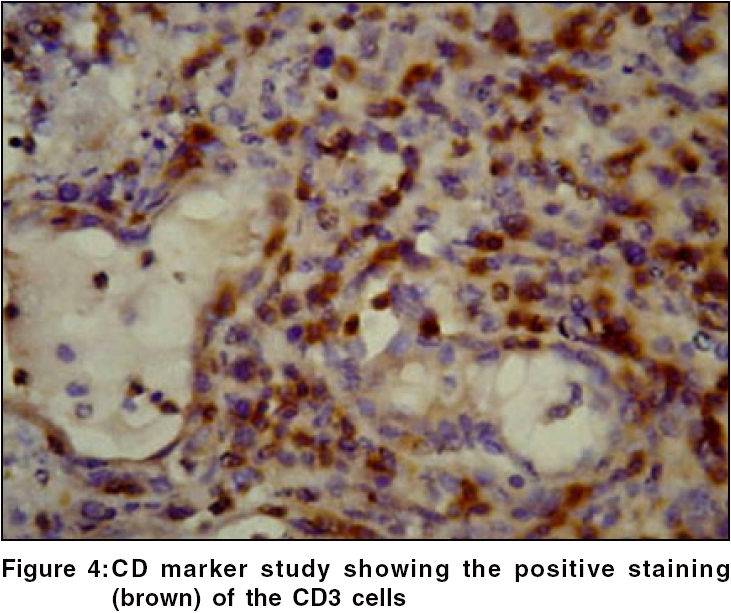
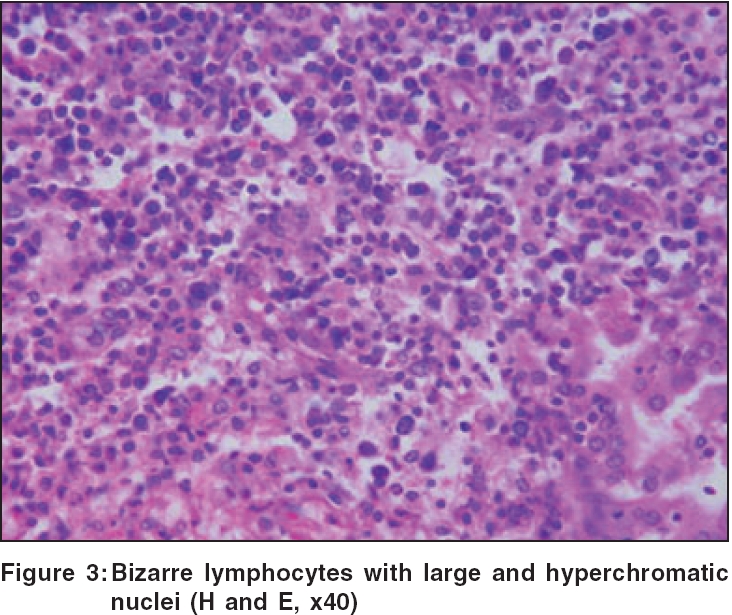
Routine hematological investigations were as follows: Hb, 14.9 gm%; CBC, 7900/mm 3 (polymorphs, 68%; lymphocytes, 22%; and eosinophils, 10%); and a markedly elevated ESR, 110 mm at the end of 1 h. Tumor marker for multiple myeloma and lymphoma- beta-2 microglobulin was negative. The level of serum LDH, one of the prognostic factors for Non Hodgkin′s lymphoma, was 229 m/l (Normal range - 100 to 190 m/l). Renal and liver function tests were within normal limits. Serology for HIV, VDRL, TPHA was negative. CT scan of the head and neck revealed a soft tissue swelling in the left nasal cavity and ipsilateral maxillary sinus, extending through the hard palate into the oral cavity [Figure - 2]. Histopathology of the mass showed abnormal lymphocytes with pleomorphic nuclei and an increased number of atypical and typical mitoses on a background of diffuse dense infiltrate of lymphocytes, plasma cells and neutrophils [Figure - 3]. Immunohistochemistry (IHC) was positive for T cell markers, i.e., CD3 [Figure - 4] and CD45RO [Figure - 5], but not for a B cell marker, i.e., CD19. IHC markers for NK cell, i.e., CD16, CD56, CD57 were unavailable. Although NK marker studies could not be done, the biologic behavior of NK positive and T positive nasal extranodal lymphomas is similar and hence they are collectively termed as NK/T-cell lymphomas. Thus, based on the clinical picture, histopathology and immunohistochemistry, we came to the diagnosis of extranodal nasal NK/T-cell lymphoma.
Accordingly, the patient was started on multidrug chemotherapy, i.e., the CHOP regimen consisting of injections of cyclophosphamide (750 mg/m 2), doxorubicin (40 mg/m 2) and vincristine (1.4 mg/m 2) and oral prednisolone (50 mg b.i.d. for 5 days) given every 3 weeks for 6 cycles. It was to be followed by involved field radiotherapy. However, the patient developed vomiting, giddiness and persistent spikes of fever after the second pulse. The cause of the fever could not be ascertained. He succumbed to the disease shortly thereafter.
Discussion
NK/T-cell neoplasms are rare, the most common and well-characterized ones being the nasal and nasal-type NK/T-cell neoplasms. Their characteristic histologic feature is an angiocentric/angiodestructive growth pattern with zonal necrosis. These tumors have a predilection for the nasal cavity and paranasal sinuses and were formerly called lethal midline granuloma or polymorphic reticulosis. ′Nasal-type′ lymphomas show the same histologic features but arise from extranasal sites such as the skin, gastrointestinal tract, testis, kidney and upper respiratory tract and rarely, the eye/orbit. Mature or peripheral NK/T-cell lymphomas account for only 10-15% of non-Hodgkin′s lymphomas (NHL) and are now classified as ′cutaneous T cell and NK cell lymphomas′ by the WHO-EORTC.[4],[9]
Amongst these, extranodal nasal NK/T-cell lymphomas are a rare variety seen mainly in Asia and Latin America. They commonly affect males with a median age of 50 years. The commonest presentation is chronic nasal obstruction; purulent rhinorrhea is the second most common symptom.[1],[5] Systemic symptoms like fever and weight loss are noticed only in advanced cases.[1],[5] Our patient had also presented with symptoms of chronic nasal obstruction and nasal discharge. It is important to note the nondescript nature of these presenting symptoms, which may result in delayed diagnosis. Apart from the nasal cavity, other sites of involvement are skin, gut, testis, kidneys, upper respiratory tract and rarely, the eyes. The skin is the most common site of involvement after the nasal cavity/nasopharynx and skin involvement may be a primary or secondary manifestation of the disease. Approximately 10-20% of nasal lymphomas also have skin involvement. Many nasal type NK/T-cell lymphomas arise in the skin. The cutaneous nasal type T-cell lymphomas occur more often in females, have a higher frequency of a T cell rather than a NK cell phenotype and are less associated with EBV. The disease is aggressive, with a median survival of 5 months for patients with cutaneous and extra-cutaneous disease.
Histologically, a mixed cellular infiltrate consisting of atypical lymphocytes, plasma cells, eosinophils, histiocytes and acute inflammatory cells is seen.[6] Majority of the tumor cells are large dysplastic cells with hyperchromatic or vesicular chromatin. Similar finding with large number of atypical lymphocytes and atypical mitoses was noted in our case. Angiocentricity and angioinvasion are generally seen (>60%), but often the picture is that of necrosis without angiocentricity. Inability to demonstrate vascular involvement may be due to a sampling error.[7] Immunohistochemistry and flow cytometry demonstrate the presence of T-cell associated markers like CD2, CD7, CD45RO, CD43.[5] However, a loss of pan T-cell markers such as CD3 is often seen. These tumors often express the natural killer cell marker CD56 but not CD16 or CD57. Immunohistochemistry in this case confirmed the T-cell lineage of the tumor by demonstrating a positive CD3 and CD45RO and negative B cell markers. Zhong et al. noted that most upper aerodigestive tract NK/T-cell lymphomas were genotypically of NK cell origin and only a few belonged to T cell lineage.[8] Approximately 80% of cases arise from true NK cells; 10-30% are T cell with γδ>αβ type.[9]
Epstein-Barr virus RNA is detected in most of these growths by in situ hybridization.[8] It is present in the majority (80-100%) of nasal NK/T-cell lymphomas and less often in nasal type NK/T-cell lymphomas (15-40% or more in some series).
CD56 is not specific only to NK cells and is expressed even on Merkel cells and cytotoxic T cell clones, which may present with a subcutaneous panniculitis-like syndrome.[10],[11] Since many entities of NK and T cell derivation do not have a specific immunophenotype, the clinical syndrome is more important in diagnosing and defining NK or T cell neoplasms than the precise cell of origin identified by immunophenotype studies.[4]
Nasal NK/T cell lymphoma in Asians follows an aggressive course, with death occurring due to local relapse or systemic spread in 50% of the cases.[5],[6] Multidrug chemotherapy (CHOP regimen) followed by involved field radiotherapy appears to be the most effective treatment approach.[6] The delay in presentation and thus the delay in diagnosis is possibly one of the reasons why our patient could not be saved in spite of installation of effective chemotherapy.
This case illustrates the unusual presentation of a rare lymphoma that presented with innocuous symptoms but followed a rapid downhill and fatal course. We could not find any other report of nasal lymphoma that presented solely as a palatal ulcer.
| 1. |
Gourin CG, Johnson JT, Selvaggi K. Nasal T-cell lymphoma: Case report and review of diagnostic features . Ear Nose Throat J 2001;80:458-60.
[Google Scholar]
|
| 2. |
Sakata K, Hareyama M, Ohuchi A. Treatment of lethal midline granuloma type nasal T-cell lymphoma. Acta Oncol 1997;36:307-11.
[Google Scholar]
|
| 3. |
Ratech H, Burke JS, Blayney DW, Sheibani K, Rappaport H. A clinicopathological study of malignant lymphomas of the nose, paranasal sinuses and hard palate, including cases of lethal midline granuloma. Cancer 1989;64:2525-31.
[Google Scholar]
|
| 4. |
Jaffe ES, Krenacs L, Raffeld M. Classification of cytotoxic T-cell and natural killer cell lymphomas. Semin Hematol 2003;40:175-84.
[Google Scholar]
|
| 5. |
Hartig G, Montone K, Wasik M, Chalian A, Hayden R. Nasal T-cell lymphoma and the lethal midline granuloma syndrome. Otolaryngol Head Neck Surg 1996;114:653-6.
[Google Scholar]
|
| 6. |
Cleary KR, Batsakis JG. Sinonasal lymphomas. Ann Otol Rhinol Laryngol 1994;103:911-4.
[Google Scholar]
|
| 7. |
Jaffe ES. Post-thymic T-cell lymphomas. In : Jaffe ES, editor. Surgical Pathology of Lymph Nodes and Related Organs. 2nd ed. W B Saunders: Philadelphia; 1995. p. 379.
[Google Scholar]
|
| 8. |
Zhong BN, Zhang XH, Li M. Study of the pathology, immunophenotype, etiology and genetic markers of NK/T-cell lymphoma. Zhonghua Xue Ye Xue Za Zhi 2003;24:505-9.
[Google Scholar]
|
| 9. |
Greer JP, Kinney MC, Loughran TP Jr. T cell and NK cell lymphoproliferative disorders. Hematology online. http://www.asheducationbook.org/cgi/reprint/2001/1/259. Last accessed : 14th March 2006.
[Google Scholar]
|
| 10. |
McNiff JM, Cowper SE, Lazova R, Subtil A, Glusac EJ. CD56 staining in Merkel cell carcinoma and natural killer-cell lymphoma: magic bullet, diagnostic pitfall, or both? J Cutan Pathol 2005;32:541-5.
[Google Scholar]
|
| 11. |
Hoque SR, Child FJ, Whittaker SJ. Subcutaneous panniculitis-like T-cell lymphoma: a clinicopathological, immunophenotypic and molecular analysis of six patients. Br J Dermatol 2003;148:516-25.
[Google Scholar]
|
Fulltext Views
3,235
PDF downloads
1,892





