Translate this page into:
Need for a well-balanced sunscreen to protect human skin from both Ultraviolet A and Ultraviolet B damage
Correspondence Address:
Dominique Moyal
25-29 Quai Aulagnier - 92665 Asnieres sur seine
France
| How to cite this article: Moyal D. Need for a well-balanced sunscreen to protect human skin from both Ultraviolet A and Ultraviolet B damage. Indian J Dermatol Venereol Leprol 2012;78:24-30 |
Abstract
Skin exposure to sunlight can cause many adverse effects. It is now recognized that both Ultraviolet A (UVA) and UVB wavelengths are responsible for the detrimental effects of solar radiation on skin. With our increasing knowledge on the harmful effects of UVA, the need for effective, well-balanced photoprotection has become more crucial. Numerous clinical studies showed that well-balanced sunscreen, with a SPF/UVAPF ratio ≤ 3, provide the most effective protection against pigmentation (especially on dark skin), DNA damage, UV-induced skin immunosuppression and photodermatoses. The calculation of UVA protection required in Asia revealed its particular importance in India, and gives clear evidence that the SPF value alone is not sufficient to evaluate the efficacy of a sunscreen.Introduction
Solar UV radiation reaching the earth is a combination of UVB (290-320 nm) and UVA (320-400 nm) wavelengths. Acute as well as chronic sun exposure is well known to induce biological and clinical damage, such as sunburn, photoaging, skin immunosuppression, photodermatoses and photocarcinogenesis. UVB rays, which include most energetic photons reaching the earth′s surface, participate in all of this damage. Although UVA rays are less energetic than UVB rays, they play a significant role in skin immunosuppression, photoaging, and mutagenesis. [1],[2],[3],[4],[5] Further, UVA accounts for at least 95% of the solar UV irradiance received at ground level. Hence, sunscreens should effectively protect against UVB as well as UVA radiation [6]
The efficacy of a sunscreen is assessed primarily by its sun protection factor (SPF). [7] Since, by definition, the SPF measures the protection against erythema, which is mainly induced by UVB wavelengths; it does not provide information on UVA photoprotection. Indeed, UVA contributes only a small percentage of the skin erythemal response. Therefore, the SPF does not reflect the efficacy of protection against all biological end-points, induced by the entire solar UV spectrum. [8] SPF is not a good measure for broad spectrum protection. Nevertheless, an effective, well-balanced photoprotection against UVB and UVA radiation seems more crucial because of our increasing knowledge of the harmful effects of UVA. [1],[2],[3],[4],[5],[6]
The different aspects of UVA induced damage discussed in this article are summarized in [Table - 1].
Because of the link between amount of products applied and efficacy, it is important to ensure by consumer education that a sufficient quantity is applied on the skin. To reach the expected protection, the quantity should be 2 mg/cm². Lower amount of product applied should be compensated by re-application.
Methods of Assessment and Criteria for UVA Efficacy
Despite the availability of reliable methods, there is no worldwide consensus on how to measure and label the level of protection against UVA. The Persistent Pigment Darkening (PPD) method is probably the most widely used method to determine UVA protection factor (UVAPF) [9],[10] since persistent pigment darkening is induced by UVA radiation and not by UVB. The UVAPF is determined similarly to the SPF on human volunteers, with the following differences: Volunteers will have a phototype, able to develop an immediate pigmentation (phototypes III and IV), a UVA source will be used instead of a complete solar simulated radiation, and PPD will be the endpoint instead of erythema. The higher the UVAPF value, the better the UVA protection. The UVAPF can be also determined using an in vitro method, which has been developed to give equivalent results to the in vivo method. [11] Another approach is the measurement of the absorbance broadness (also called the critical wavelength method). [11] This method only relies on the shape of the UV absorption spectrum and not on its amplitude. Consequently, it does not evaluate the level of UVA protection: It ensures that products absorb in the long UVA waveband.
In 2006, a minimum requirement for UVA efficacy of sunscreens was chosen in Europe. [12] A UVAPF of at least 1/3 of the SPF of the product is now required, which is equivalent to a ratio SPF/UVAPF ≤3. This criterion has also been recently adopted by Australia and the Mercosur countries (viz. Argentina, Brazil, Paraguay and Uruguay). This value has been chosen based on the calculations presented in [Table - 2], linking real UV exposure to related visible biological phenomena. In humans, a UVA dose of 15 J/cm² can induce several biological signs of acute or chronic damage, [1],[2] implying that this dose should not be attained. Indeed, UVA radiation at 15 J/cm², which is reached in about 45 minutes of sun exposure, is able to induce the persistent pigment darkening (PPD) phenomenon, which is an oxidation of the melanin. This PPD is induced very easily on dark skin (III, IV, V).
Since most Indians have Fitzpatrick skin phototypes IV to V, [13] these calculations suggest that the SPF/UVAPF ratio of a sunscreen should be < 3 in India too. It is also important to note that UVA damage can occur after an acute or repeated UVA exposure below 15 J/cm².
Prevention of Excessive Pigmentation Induced by UV Exposure
Sun exposure induces the UVA and UVB pigmentation phenomena. UVA-induced changes begin with immediate pigment darkening (IPD), which fades rapidly. However, residual pigmentation, called persistent pigment darkening (PPD), may persist for many weeks depending on the UVA dose and skin type. Neo-melanization or delayed pigmentation, which is a long-lasting (several months) tan, starts some days after UVA exposure. It is due to an increased melanin synthesis in response to intense UVA exposure or repeated suberythemal doses. [2]
UVB-induced tanning is a delayed pigmentation due to melanin synthesis. It generally appears 2-3 days after sunburn and usually disappears with epidermal turnover after 1 month. It results in a homogeneous color, which can provide some natural protection. However, and particularly in Asian skin, sun exposure can induce irregular pigmentation, hyperpigmented areas and contribute to melasma. Pigmentary changes are observed as the major sign of skin photoaging in Asians. [14],[15],[16] In darker-skinned individuals, UVA has greater pigmenting effects than UVB. [17]
The use of sunscreens or daily protection products can prevent hyperpigmentation. Well-balanced photoprotection has been shown to prevent hyperpigmentation in Asian skin (phototypes III, IV, V). In one study, 6 different sunscreen products, containing UVA + UVB absorbers with different SPF/UVAPF ratios, were tested [6] on volunteers′ skin exposed to solar radiation mimicking standard daily UVR. [18],[19] The in vivo protection against UVB- and UVA-induced pigmentation was assessed by determining the Pigmentation Protection Factor (PPF). [20] The SPF was determined using the international SPF test method, [7] and the UVA protecting factor (UVAPF) was measured by the PPD method. [10] The results [Table - 3] showed that products having well-balanced UVB and UVA protection [SPF/UVAPF (PPD) ≤ 3] provided higher protection against pigmentation in Asian skin. For the same level of SPF, products having the highest UVAPF had the highest PPF, and products having a SPF/UVAPF ratio below 3 were more effective than those with a ratio above 3 [Figure - 1].
 |
| Figure 1: Efficacy of two sunscreen products with the same SPF but different UVA PF in the prevention of pigmentation induced by UV light (a: product with SPF 50 UVAPF 13; b: Product with SPF 50 UVAPF 21). Product a with a well-balanced photoprotection is clearly more efficient |
Efficacy Against DNA Damage
UV-induced DNA damage activates the p53 tumor suppressor gene, which produces a very important protein that protects cells from malignant transformation. Thus, p53 protein expression following UV exposure is a sensitive biological endpoint for the evaluation of sunscreen efficacy against damage that may lead to skin cancer. It has been demonstrated that p53 can be induced after a single solar simulated radiation (SSR) dose of 0.5 MED or after a single UVA dose of 30 J/cm². [2] Multiple UVA exposure at 12.5 J/cm² (about 1 hour under zenithal sun exposure conditions) also induced p53 protein, [21] demonstrating the contribution of UVA radiation into the DNA damage. One study compared the level of protection against p53 accumulation by 2 sunscreen products having the same SPF (25) but different UVAPF in human volunteers under outdoor sun exposure conditions. [8] One product contained a potent UVA filtering system (Mexoryl® SX, Mexoryl® XL, Avobenzone) providing a UVAPF of 14, measured by the PPD method while the other had a UVAPF of 6. The volunteers applied a realistic amount of product (0.8 mg/cm 2 ) and were exposed to the sun daily for 6 days with a duration of exposure and UV dose increasing from 3 hours (6 MED, 40 J/cm² of UVA) to 6 hours (10 MED, 70 J/cm²). Although both sunscreens provided a similar level of protection against erythema, the sunscreen with well-balanced UV protection (SPF 25/UVAPF 14 = 1.8) was much more effective in protecting against p53 accumulation, demonstrating the importance of UVA protection [Figure - 2].
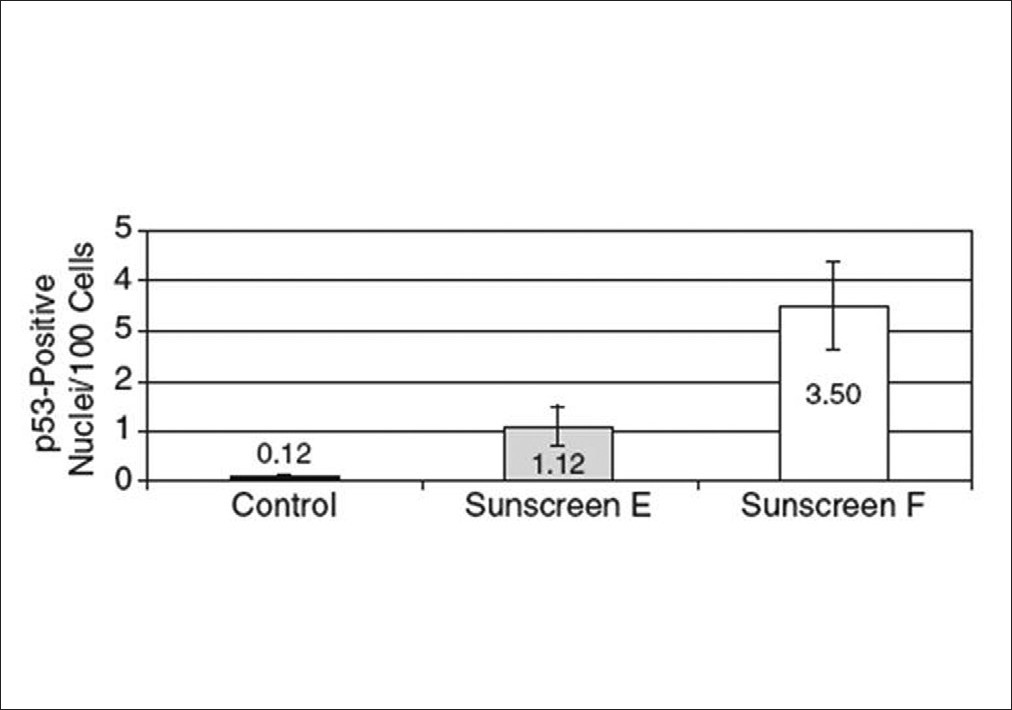 |
| Figure 2: p53 accumulation after repeated sun exposure of human skin protected by sunscreen E (SPF 25 UVA-PF 14, ratio = 1.8 ) and sunscreen F (SPF 25 UVA-PF 6, ratio = 4.2).[8] Results are means +/-SEM. UV: Ultraviolet, SPF: Sun protection factor, UVAPF: UVA protection factor |
Protection of the Skin Immune System
Exposure of human skin to UV radiation induces local immunosuppression. Both UVA and UVB are immunosuppressive. [3],[22],[23],[24] The process is thought to involve Langerhans cells (LC), the epidermal dendritic cells that are pivotal in antigen presentation.
The protective effect of sunscreens on UV-induced immune suppression has been demonstrated, and the importance of an effective protection against UVA has been stressed. The protective potential of 2 sunscreens, having the same SPF (25) but widely different level of UVA protection (UVAPF 14 vs. 6), mentioned above for the prevention of DNA damage, has been compared in vivo in conditions of outdoor exposure. [8] The results showed that both sunscreens only partially prevented the reduction of LC density and morphological alteration induced by repeated solar-simulated radiation exposure [Table - 4]. However, a significantly lower level of LC damage was seen in the area protected by the sunscreen with higher UVAPF. This again demonstrates the need for effective, balanced protection with a SPF/UVAPF ratio ≤ 3 to prevent the impairment of immune competent cells.
The delayed-type hypersensitivity (DTH) response to recall antigens was also studied as an endpoint to assess the protective role of sunscreens against skin immunosuppression, induced by UV exposure. An acute and repeated exposure to UVA induced a significant decrease in DTH response. The efficacy of sunscreens with different levels of UVA protection has been evaluated under both solar-simulated radiation and outdoor real-life exposure conditions. [23],[25] The results confirm the importance of well-balanced photoprotection using the SPF/UVAPF ratio ≤ 3 criterion. When products with same SPF have been compared, the product with the higher UVAPF and with SPF/UVAPF ratio ≤ 3 always afforded a significant higher protection against photoimmunosuppression compared to the product having the same SPF but lower UVAPF (SPF/UVAPF ≥ 3). It is also important to notice that in this study, some products with insufficient UVA protection level, had a critical wavelength value of at least 370 nm. These results demonstrated that as the sole criterion for UVA efficacy (as requested recently by the US FDA [26] ), the critical wavelength of at least 370 nm is not sufficient, and only the criterion SPF/UVAPF ≤ 3 is a good indicator of an efficacy. [6]
Protection Against Photodermatoses
Photosensitivity is a general term that designates an abnormal reaction to sunlight including phototoxicity, photoallergy and photodermatoses. The wavelengths that cause those skin abnormal reactions to sunlight mainly lie in the UVA range.
The most common photodermatosis, polymorphous light eruption (PMLE), has been particularly studied. This eruption generally appears after 1 or 1 days of sun exposure and consists of papules, reticulated erythema, vesicles and pruritus. The preventive efficacy of sunscreen products on PMLE has been demonstrated. [27] An outdoor study was performed to compare the efficacy of 2 sunscreen products with a similar high SPF (60) but with different UVA protection levels (UVAPF 28 vs 17), and consequently, different SPF/UVAPF ratios (2.1 and 3.5, respectively). [6] It was carried out under natural sunlight using realistic conditions of exposure in 10 women prone to PMLE. The UVAPF 28 sunscreen provided better PMLE prevention than the UVAPF 17 one [Figure - 3].
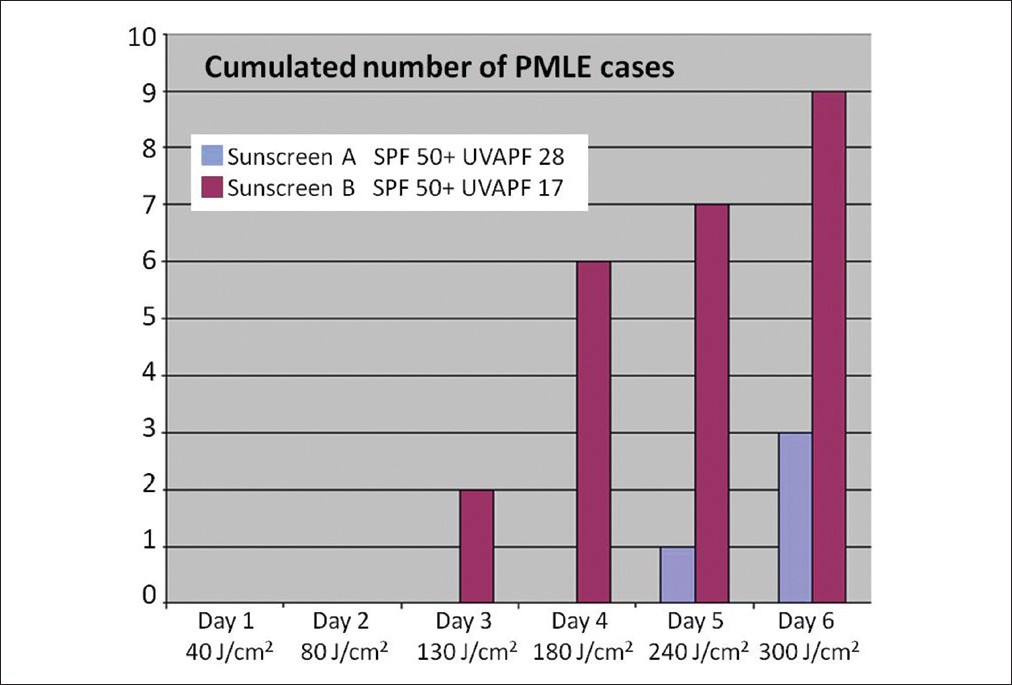 |
| Figure 3: Comparison of two high SPF 50+ products with different level of UVA protection: UVA-PF 28 sunscreen (blue bar) vs.UVA-PF 17 sunscreen (purple bar) in preventing PMLE reactions (outdoor study). Number of patients experiencing PMLE according to cumulative UVA dose. PMLE: Polymorphous light eruption |
Calculation of the UVA Protection Factor Levels Needed in Asia According to the Season
The calculation of a UVAPF ′cap′ has been based on the level of protection needed to limit the effect of UVA radiation on the skin to a level of one minimal pigmenting dose (MPD), typically equivalent to a dose of 15 J/cm 2 of UVA radiation. This UVA dose has been demonstrated as the threshold of much UVA-induced damage. The calculations have been made based on meteorological daily dose according to the season and weighed by different factors such as skin type, anatomical skin area, realistic conditions of sunscreen use and realistic duration of exposure to UVR. The resulting figures indicate the high level of UVA protection required in Asia [Figure - 4]. In India, the minimum UVAPF needed is 12-17 in winter, and the maximum is 29-30 in summer, which raises the need for a well-balanced UVB-UVA protection.
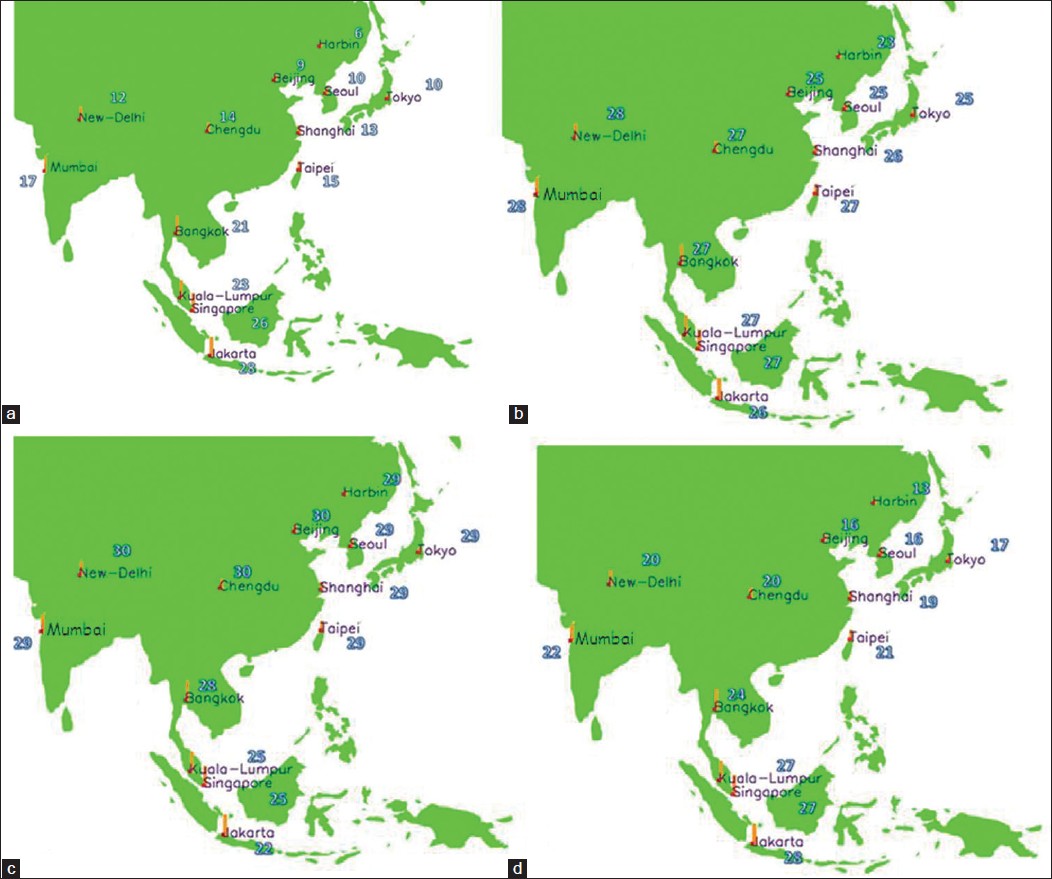 |
| Figure 4: UVA protection level required in Asia in January (a), April (b), July (c), and October (d) |
The level of UVB/UVA protection should be adapted to the consumers needs i.e. depending on the time spent outdoor during the day.
Conclusion
Both UVB and UVA play a major role in the detrimental effects of solar radiation on skin. An effective, well-balanced photoprotection, combining high UVB and UVA efficacy, appears pivotal because of increasing knowledge of the harmful effects of UVA. This review demonstrates the importance of UVA protection and gives clear evidence that the SPF value alone is not sufficient to evaluate the efficacy of a sunscreen in protecting against all biological end-points, which are the hallmarks of damage induced by the solar UV spectrum. A well-balanced sunscreen, with a SPF/UVAPF ratio ≤ 3, appears to provide the most effective protection against pigmentation (especially on dark skin), DNA damage, skin photoimmunosuppression and photodermatoses. This type of products should be available for all consumers, and recommendations to them should be done to use regularly these products to be well protected against skin UV-induced damage.
Acknowledgements
I thank François Christiaens for the calculations of the UVA protection factors required according to the season in Asia.
| 1. |
Moyal D, Fourtanier A. Acute and chronic effects of UV on skin. In: Rigel DS, Weiss RA, Lim HW, Dover JS, editors. Photoaging. New York: Marcel Dekker; 2004. p. 15-32.
[Google Scholar]
|
| 2. |
Seité S, Fourtanier A, Moyal D, Young AR. Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: A review. Br J Dermatol 2010;163:903-14.
[Google Scholar]
|
| 3. |
Damian DL, Barnetson RS, Halliday GM. Low-dose UVA and UVB have different time courses for suppression of contact hypersensitivity to a recall antigen in humans. J Invest Dermatol 1999;112:939-44.
[Google Scholar]
|
| 4. |
Lavker RM, Gerberick GF, Veres D, Irwin CJ, Kaidbey KH. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol 1995;32:53-62.
[Google Scholar]
|
| 5. |
Lowe NJ, Meyers DP, Wieder JM, Luftman D, Borget T, Lehman MD, et al. Low doses of repetitive ultraviolet A induce morphologic changes in human skin. J Invest Dermatol 1995;105:739-43.
[Google Scholar]
|
| 6. |
Fourtanier A, Moyal D, Seite S. UVA filters in sun-protection products: Regulatory and biological aspects. Photochem Photobiol Sci 2012;11:81-9.
[Google Scholar]
|
| 7. |
COLIPA, JCIA, CTFA SA: International sun protection factor (SPF) test method. Brussels: COLIPA; 2006.
[Google Scholar]
|
| 8. |
Fourtanier A, Bernerd F, Bouillon C, Duval C, Marrot L, Moyal D, et al. Protection of skin biological targets by different types of sunscreens. Photodermatol Photoimmunol Photomed 2006;22:22-32.
[Google Scholar]
|
| 9. |
Moyal D, Chardon A, Kollias N. UVA protection efficacy of sunscreens can be determinated by the persistent pigment darkening (PPD) method (Part 2). Photodermatol Photoimmunol Photomed 2000;16:250-5.
[Google Scholar]
|
| 10. |
Japan Cosmetic Industry Association (JCIA). Measurements standard for UVA protection efficacy. Tokyo, Japan: JCIA; 1996.
[Google Scholar]
|
| 11. |
Moyal D. UVA protection labeling and in vitro testing methods. Photochem Photobiol Sci 2010;9:516-23.
[Google Scholar]
|
| 12. |
European Commission recommendation. On the efficacy of sunscreen products and the claims made relating thereto. Official J Eur Union 2006;265;39-43.
[Google Scholar]
|
| 13. |
Rai VM, Shenoi SD, Balachandran C, Pai S. Minimal erythema response (MED) to solar simulated irradiation in normal Indian skin. Indian J Dermatol Venereol Leprol 2004;70:277-9.
[Google Scholar]
|
| 14. |
Zhao P, Zhu X, Liu Y, Wang B, Wang C, Burns FJ. Solar ultraviolet radiation and skin damage: An epidemiological study among a Chinese population. Arch Environ Health 1998;53:405-9.
[Google Scholar]
|
| 15. |
Chung JH. Photoaging in Asians. Photodermatol Photoimmunol Photomed 2003;19:109-21.
[Google Scholar]
|
| 16. |
Goh SH. The treatment of visible signs of senescence: The Asian experience. Br J Dermatol 1990;122 Suppl 35:S105-9.
[Google Scholar]
|
| 17. |
Kollias N, Malallah YH, al-Ajmi H, Baqer A, Johnson BE, González S. Erythema and melanogenesis action spectra in heavily pigmented individuals as compared to fair-skinned Caucasians. Photodermatol Photoimmunol Photomed 1996;12:183-8.
[Google Scholar]
|
| 18. |
Christiaens FJ, Chardon A, Fourtanier A, Frederick JE. Standard ultraviolet daylight for nonextreme exposure conditions. Photochem Photobiol 2005;81:874-8.
[Google Scholar]
|
| 19. |
Seité S, Medaisko C, Christiaens F, Bredoux C, Compan D, Zucchi H, et al. Biological effects of simulated ultraviolet daylight: A new approach to investigate daily photoprotection. Photodermatol Photoimmunol Photomed 2006;22:67-77.
[Google Scholar]
|
| 20. |
Moyal D. Prevention of ultraviolet-induced skin pigmentation. Photodermatol Photoimmunol Photomed 2004;20:243-7.
[Google Scholar]
|
| 21. |
Séité S, Moyal D, Verdier MP, Hourseau C, Fourtanier A. Accumulated p53 protein and UVA protection level of sunscreens. Photodermatol Photoimmunol Photomed 2000;16:3-9.
[Google Scholar]
|
| 22. |
LeVee GJ, Oberhelman L, Anderson T, Koren H, Cooper KD. UVA II exposure of human skin results in decreased immunization capacity, increased induction of tolerance and a unique pattern of epidermal antigenpresenting cell alteration. Photochem Photobiol 1997;65:622-9.
[Google Scholar]
|
| 23. |
Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from suppression of the elicitation phase of delayed-type hypersensitivity response in humans. J Invest Dermatol 2001;117:1186-92.
[Google Scholar]
|
| 24. |
Seité S, Zucchi H, Moyal D, Tison S, Compan D, Christiaens F, et al. Alteration in human epidermal Langherans cells by ultraviolet radiation: Quantitative and morphological study. Br J Dermatol 2003;148:219-9.
[Google Scholar]
|
| 25. |
Moyal DD, Fourtanier AM. Efficacy of broad-spectrum sunscreens against the suppression of elicitation of delayed-type hypersensitivity responses in humans depends on the level of ultraviolet A protection. Exp Dermatol 2003;12:153-9.
[Google Scholar]
|
| 26. |
Federal Register, Food and Drug administration (USA). Department of Health and Human Services. Labeling and Effectiveness testing: Sunscreen drug products over-the -counter Human Use. 2011. p. 201-310.
[Google Scholar]
|
| 27. |
Fourtanier A, Moyal D, Seité S. Sunscreens containing the broad-spectrum UVA absorber, Mexoryl SX, prevent the cutaneous detrimental effects of UV exposure: A review of clinical study results. Photodermatol Photoimmunol Photomed 2008;24:164-74.
[Google Scholar]
|
Fulltext Views
5,512
PDF downloads
1,478





