Translate this page into:
Need to restage Korean melanoma patients following publication of the 8th edition of the American Joint Committee on Cancer staging manual
Corresponding author: Prof. Jun Young Kim, Department of Dermatology, Kyungpook National University Hospital, Daegu, South Korea. 198kjy@hanmail.net
-
Received: ,
Accepted: ,
How to cite this article: Lee HJ, Park KD, Jang YH, Lee WJ, Lee SJ, Kim JY. Need to restage Korean melanoma patients following publication of the 8th edition of the American Joint Committee on Cancer staging manual. Indian J Dermatol Venereol Leprol 2022;88:332-6.
Abstract
Background:
The tumor, nodes and metastasis (TNM) classification and stage grouping have been updated in the 8th edition of the American Joint Committee on Cancer (AJCC) melanoma staging manual. However, restaging all the previous cases are not recommended.
Aims:
The aims of the study were to investigate the necessity of restaging Korean melanoma patients staged by the previous edition of the AJCC manual.
Methods:
Differences in the staging criteria of the 7th and 8th editions of the AJCC manual were identified. The staging of 276 primary melanomas from January 2011 to December 2018 was classified by both 7th and 8th editions of the manual and their differences were compared.
Results:
Staging by 7th and 8th edition of the AJCC manual differed in 64 cases (23.2%). The pathological prognostic staging changed in 35 (12.7%), and 29 (10.5%) had changes in only TNM classification but not the pathological staging. None of the patients needed additional sentinel lymph node biopsy or systemic treatment as a result of restaging. Additional counseling was needed for the patients, because melanoma-specific survival was increased in the 8th edition.
Limitations:
This is a retrospective study with relatively small number of patients at a single tertiary center in Korea.
Conclusion:
Assessment of the need for additional sentinel lymph node biopsy or systemic treatment is recommended because of the latest changes in the AJCC melanoma staging manual. Although the restaging of previously staged melanomas is not significantly needed in our patients, still the differences in TNM classification and/or pathological prognostic staging suggest the need to separately recognize the patients previously staged by 7th edition and recently staged by 8th edition. Careful counseling about melanoma-specific survival is needed for Asian patients.
Keywords
American Joint Committee on Cancer
melanoma
staging
survival
TNM classification
Introduction
The 8th edition of the American Joint Committee on Cancer (AJCC) melanoma staging manual was implemented in January 2018.1 The tumor, nodes and metastasis (TNM) classifications and stage grouping have become more complicated, but the deficiencies of the previous 7th edition which was in use since 2010 have been improved.2 Accurate staging is essential for the assessment of prognosis and rational treatment decisions. As the changes in the 8th edition compared with 7th edition are substantive, this study investigated the need to restage previously staged melanoma patients in Korea and to determine whether restaging results in need of additional diagnostic tests or systemic treatment.
Methods
The medical records and clinical imaging of 405 melanoma patients treated at Kyungpook National University Hospital, a single tertiary center in Korea between January 2011 and December 2018 were retrospectively reviewed. After excluding melanomas of non-cutaneous origin, unknown primary melanomas and the cases that lacked data needed for staging, 275 patients with 276 primary melanomas were included in the analysis. One patient had two primary sites, one on the left ear helix and the other on the right sole. Before restaging the patients, we carefully compared the staging criteria of the 7th and 8th editions of the AJCC. The nodes and metastasis classification and pathological prognostic staging of 276 primary melanomas based on their initial staging evaluation were determined by both 7th and 8th editions and their differences were compared. The study was approved by the institutional review board (KNUH 2020-03-027).
Results
Comparison of the 7th and 8th editions of the American Joint Committee on Cancer manual
Major revisions and the details affecting melanoma staging made in 8th edition of the AJCC manual are summarized in Table 1.1-3 Moreover, the 18 practical changing points of difference between the 7th and 8th editions are summarized in Table 2. The T classification has been revised to restage patients with Breslow thickness (BT) of 0.75–1.0 mm without ulceration and mitoses of <1/mm2 as T1b rather than T1a. They were not indicated for sentinel lymph node biopsy in the 7th edition. The 8th edition recommends that clinicians “discuss and consider” sentinel lymph node biopsy for T1b patients. Six (2.2%) patients in our study were affected by this change [Table 2]. Three (1.1%) of these had negative sentinel lymph node biopsy, and in the other three sentinel lymph node biopsy was not done but they were only followed-up. The 8th edition defines “MSI” as any intra-lymphatic metastasis such as satellites, microsatellites and in-transit metastasis, or local recurrence [Table 1]. Hence, in the N classification, the stage of patients with local recurrence and no metastatic regional nodes was changed from N0 to N1c which is a clinically significant upstaging over Stage III which needs systemic treatments. Two patients (0.7%) were affected by this change [Table 2]. One was upstaged from Stage IIB (T4aN0M0) to Stage IIIC (T4aN1cM0). The other was already Stage IV with distant metastasis and received adjuvant systemic treatments. The change of stage was from T2bN0M1c to T2bN1cM1c.
| Classifications | Details |
|---|---|
| T classification | T1 definition is revised by BT of 0.8mm and presence of ulceration Mitotic index has been removed from the criteria BT is recorded to the nearest 0.1 mm, not 0.01 mm |
| N classification | New descriptors of regional node metastasis, “clinically occult” and “clinically detected” replaced previous “microscopic” and “macroscopic” The term “MSI” includes any ILM such as satellites, microsatellites and in-transit metastasis, or local recurrence The presence of MSI refers to N1c, N2c and N3c depending on the number of metastatic regional lymph nodes |
| M classification | Distant metastasis to the central nervous system is subcategorized as M1d LDH elevation no longer upstages to M1c and is added to each M1 subcategory |
| Pathologic prognostic staging | Stage IA includes T1bN0M0 which was previously stage IB in the 7th edition The T and N classifications of pathological Stage III groups has been revised to include four subgroups (Stage IIIA-D) Stage IIID includes T4b(thick and ulcerated primary) and advanced regional nodal and/or ILM and/or local recurrence |
BT: Breslow thickness, MSI: Satellites, in-transit metastasis or local recurrence, ILM: Intra-lymphatic metastasis, LDH: Lactate dehydrogenase
| Changing points | 7th AJCC | 8th AJCC | This study (n [%])* | |
|---|---|---|---|---|
| T classification | BT ≤0.74 mm w/o ulcer and mitoses ≥1/mm2 | T1b | T1a | 2 (0.7) |
| BT 0.75–1.0 mm w/o ulcer and mitoses <1/mm2 | T1a | T1b | 6 (2.2) | |
| BT 1.01–1.04 mm | T2 | T1 | ||
| BT 2.01–2.04 mm | T3 | T2 | ||
| BT 4.01–4.04 mm | T4 | T3 | ||
| N classification | Satellites or in-transit metastasis w/o regional nodes | N2c | N1c | 12 (4.3) |
| Satellites or in-transit metastasis with 1 node | N3 | N2c | 4 (1.4) | |
| Satellites or in-transit metastasis with two or more nodes | N3 | N3c | 4 (1.4) | |
| Local recurrence w/o regional nodes | N0 | N1c | 2 (0.7) | |
| Local recurrence with one node | N1a/N1b | N2c | 1 (0.4) | |
| Local recurrence with 2–3 nodes | N2a/N2b | N3c | 1 (0.4) | |
| Local recurrence with four and more nodes | N3 | N3c | 2 (0.7) | |
| Four or more nodes, all clinically occult, no MSI | N3 | N3a | 4 (1.4) | |
| Four or more nodes, ≥ clinically detected, no MSI | N3 | N3a | 5 (1.8) | |
| M classification | Distant metastasis to the central nervous system | M1c | M1d | 7 (2.5) |
| Any distant metastasis with elevated LDH level | M1c | M1a-d (1) | 5 (1.8) | |
| Pathological prognostic staging within same TNM | T1bN0M0 | Stage IB | Stage IA | 5 (1.8) |
| T3/4 with any N | Upstaging within stage III grouping | 14 (5.1) | ||
Distribution of melanoma staging by the 7th and 8th editions of American Joint Committee on Cancer manual
Out of 276 primary melanomas, 64 cases (23.2%) were changed by the revision in the 8th edition of American Joint Committee on Cancer manual. The final pathological prognostic staging changed in 35 cases (12.7%). The nodes and metastasis classification changed in the other 29 patients (10.5%) without any change in the pathological staging [Table 3].
| TNM classification | Pathological prognostic staging | n (%)* |
|---|---|---|
| Any differences between 7th AJCC and 8th AJCC | 64 (23.2) | |
| Changed | Same | 29 (10.5) |
| Same | Changed | 19 (6.9) |
| Changed | Changed | 16 (5.8) |
| Same | Same | 212 (76.8) |
| Total | 276 (100) | |
The distribution of the pathological prognostic staging of the 276 primary melanomas according to each edition, including 35 cases (12.7%) with differences, are shown in Figure 1. Seven cases were downstaged and 28 cases were upstaged when re-classified from 7th edition to 8th edition. Seven cases (2.5%) changed from Stage IB to Stage IA including two cases from T1b to T1a by the T1 redefinition. The other five cases had same nodes and metastasis classification (T1bN0M0) that was Stage IB by the 7th edition, but became Stage IA by the 8th edition.
Stage III was the most affected group with 27 cases (9.8%) increased to Stage IIIB–IIID. Fourteen (5.1%) had the same nodes and metastasis classification by both editions, but the pathological staging of T3/4 with any N (metastatic regional lymph nodes or presence of MSI) was revised to upstaging within Stage III grouping even if the nodes and metastasis classification remained same as previous edition. Consequently, two cases of Stage IIIA (T3aN2aM0 and T4aN1aM0) became Stage IIIB (T3aN2aM0) and Stage IIIC (T4aN1aM0). The remaining 12 cases of Stage IIIB with over T3b became Stage IIIC. The N classification of 13 of the 27 cases (4.7%) changed. In the 7th edition, satellites or in-transit metastasis without metastatic regional lymph nodes was classified as N2c. In the 8th edition, MSI including local recurrence refers to N1c, N2c and N3c. Owing to this, five cases (1.8%) changed from Stage IIIA/B to Stage IIIC. In the 8th edition, the N3 classification was subcategorized to N3a-c, and T4b with N3a-c were newly categorized to Stage IIID. Due to this, eight cases (2.9%) changed from Stage IIIC to Stage IIID [Figure 1]. There was only one case (0.4%) restaged from below Stage II to over Stage III. The patient had a local recurrence without metastatic regional lymph nodes, so Stage IIB (T4aN0M0) by the 7th edition increased to Stage IIIC (T4aN1cM0) by the 8th edition [Figure 1].
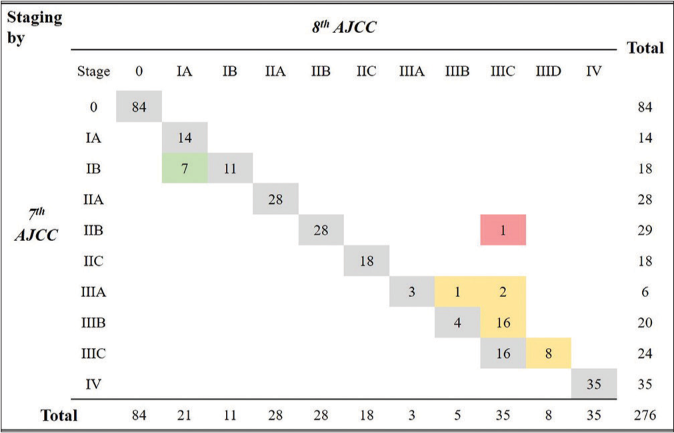
- Distribution of pathological staging by 7th and 8th AJCC. Among total 276 cases, 35 (12.7%) showed the differences. Green box (seven cases) downstaged from Stage IB to IA. Yellow box (27 cases) upstaged to Stage IIIB-IIID within Stage III. Red box presents the only one case restaged from below Stage II to over III
Discussion
In the 7th edition, sentinel lymph node biopsy was indicated for all primary melanomas with BT ≥1 mm and concurrent normal regional lymph nodes by physical examination.4,5 The 7th edition also included a consensus recommendation of sentinel lymph node biopsy for T1 melanomas with ulceration, mitoses ≥1 mm2 and/or Clark level IV/V invasion, especially if BT exceeded 0.75 mm. Those criteria, rather than only tumor thickness, were relevant for patients without significant comorbidities, younger than 40–45 years of age, or with an uncertain tumor depth because of deep tumor-positive margins in biopsy specimens.4 But now, the 8th edition recommends that physicians “discuss and consider” sentinel lymph node biopsy for T1b Stage IA and “discuss and offer” sentinel lymph node biopsy for Stage IB.6 So, we wondered whether the changes would result in additional needs of sentinel lymph node biopsy by restaging. Following the 8th edition, patients with BT 0.75–1.0 mm without ulceration, and with mitoses <1/mm2 were restaged from T1a to T1b [Table 2]. They were not indicated for sentinel lymph node biopsy by the 7th edition, but the 8th edition indicated a need to “discuss and consider” sentinel lymph node biopsy. Six cases (2.2%) of our study were affected by this change. Three (1.1%) had sentinel lymph node biopsys by clinical decision and all had negative results. Two had favorable clinical status and were just monitored. One was diagnosed as lentigo maligna melanoma and was just monitored, because it would have been difficult to perform sentinel lymph node biopsy of the site in the head and neck melanomas. None of the six patients had poor outcome with regional or distant metastasis. Their T1b restaged by 8th edition indicates a need to consider sentinel lymph node biopsy. Although a small percentage of patients were restaged due to this change, the consequences of the difference and potential impact on patient care need to be considered.
The analysis included the possible need for adjuvant systemic treatments after restaging. The risk of loco regional and/or distant metastasis is substantial in patients with resected high-risk melanoma (Stage IIB/C-III) because of the presence of undetected hematogenous micrometastasis at the time of diagnosis.7 As melanoma-specific survival decreases considerably above Stage IIB [Table 4], such patients are at high-risk and need systemic adjuvant treatements.1,2,8 However, the most of the restaging in our patients did not result in changes from Stage I-IIA to high-risk Stage IIB/CIII. The majority of changes (27/35) occurred in patients who were under Stage III groupings by both editions [Figure 1] and were already given systemic treatments. However, as we pointed out in Table 2, local recurrence without regional node metastasis results restaging from N0 (below Stage II) to N1 (above Stage III). Local recurrence is commonly caused by dermal lymphatic metastasis near the resection scar of primary tumor, while satellite and in-transit metastasis by endolymphatic spread.9 This point requires careful consideration to indicate systemic treatments for previously diagnosed patients. Two of our patients (0.7%) changed from N0 to N1 because of the presence of local recurrence [Table 2]. One case was an increase from Stage IIB (T4aN0M0) to Stage IIIC (T4aN1cM0). This change indicated systemic treatments after wide excision of the primary tumor, but further sentinel lymph node biopsy and systemic treatments were skipped because of the patient’s old age. Another case was in Stage IV (from T2bN0M1c to T2bN1cM1c) with distant metastasis, already being given adjuvant systemic treatments.
Major changes have been made in the 8th edition and the proportion of differences in nodes and metastasis classification and pathological prognostic staging in our study are shown in Table 3. The staging of 64 cases (23.2%) out of 276 primary melanomas was changed by the revisions. Thirty-five cases (12.7%) had changes in pathological prognostic staging that could potentially influence the prognosis of the disease and require careful decisions and counseling. These differences in nodes and metastasis classification and/or pathological prognostic staging suggest that we should separately recognize the patients previously staged by 7th edition and recently staged by 8th edition.
Considering above changes, counseling patients on disease prognosis should be carefully reviewed following the revised melanoma-specific survival by the 8th edition of the AJCC manual. The five-year melanoma-specific survival is improved in the 8th edition compared with that of similar stages in the 7th edition [Table 4].1,2 Because of recently approved immunologic checkpoint inhibitors and targeted antitumor therapies, the five-year melanoma-specific survival of high-risk Stage (IIB/C-III) patients is significantly improved.10,11 The adoption of these new treatments may have influenced melanoma-specific survival in general, but Asian melanoma patients still have relatively poorer prognosis than Caucasian patients.12,13 This may be due in part to the fact that these new treatment modalities are very costly and are neither affordable to many patients nor available in some hospitals in parts of Asia. Besides, acral lentiginous melanoma is the most common subtype in Asian patients. This type is more likely to have thicker primary tumors with an advanced stage at the time of diagnosis and respond poorly to checkpoint inhibitors.14 Fujisawa et al.8 reported that the five-year mean melanoma-specific survival of 3097 patients in Japan (calculated mean: 76.2%) was lower than that in the 8th edition database (calculated mean: 81.7%) in most of stage groups [Table 4]. The results showed that the melanoma-specific survival appeared to be more disparate from the 8th edition in advanced stages from Stage IIC onward [Table 4]. The gap between 8th edition and the study data ranges from 5.5% to 13.7% through each staging above Stage IIC [Table 4]. However, like the 8th edition, the melanoma-specific survival considerably decreases above Stage IIB. Consequently, it suggests the limitations of applying international AJCC data to Asian melanomas.
| Stage | 5-year MSS (%) | ||
|---|---|---|---|
| 7th AJCC | 8th AJCC | Fujisawa et al.8 (by 8th AJCC) | |
| IA | 97 | 99 | 97.9 |
| IB | 91–94 | 96 | 96.2 |
| IIA | 79–82 | 94 | 94.1 |
| IIB | 68–71 | 87 | 84.4 |
| IIC | 53 | 82 | 72.2 |
| IIIA | 78 | 93 | 87.5 |
| IIIB | 59 | 83 | 72.6 |
| IIIC | 40 | 69 | 55.3 |
| IIID | 32 | 26.0 | |
| Calculated mean* | 71.2 | 81.7 | 76.2 |
Limitations
The interpretation of our results may be limited by the retrospective analysis, relatively small number of patients diagnosed and treated at a single center in Korea, and lack of follow-up data and survival data. Further studies are needed to validate the impact of the 8th edition of the AJCC manual in Asian melanoma patients. Moreover, to confidently use newly revised staging criteria, the necessity of restaging should be verified in Caucasian melanoma patients.
Conclusion
An assessment of the need for additional sentinel lymph node biopsy or systemic treatment is recommended because of the latest changes in the AJCC melanoma staging manual. Sentinel lymph node biopsy needs to be considered for patients with breslow thickness 0.75–1.0 mm without ulceration and with mitoses <1/mm2. Local recurrence might now be an indication for systemic treatments. Although the restaging of previously staged melanomas is not significantly needed in our patients, still the differences in nodes and metastasis classification and/or pathological prognostic staging suggest the need to separately recognize the patients previously staged by 7th edition and recently staged by 8th edition. Although melanoma-specific survival has considerably improved, careful counseling about melanoma-specific survival is needed for Asian patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2018R1C1B5085905).
Conflict of interest
There are no conflict of interest.
References
- Melanoma staging: Evidence-based changes in the American joint committee on cancer 8th edition, cancer staging manual. CA Cancer J Clin. 2017;67:472-92.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical implications of the 8th edition of the American joint committee on cancer melanoma staging. J Surg Oncol. 2019;119:168-74.
- [CrossRef] [PubMed] [Google Scholar]
- Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-206.
- [CrossRef] [PubMed] [Google Scholar]
- Sentinel node biopsy and standard of care for melanoma. J Am Acad Dermatol. 2009;60:872-5.
- [CrossRef] [PubMed] [Google Scholar]
- Current controversies in early-stage melanoma: Questions on management and surveillance. J Am Acad Dermatol. 2019;80:15-25.
- [CrossRef] [PubMed] [Google Scholar]
- Should sentinel lymph node biopsy be performed for all T1b melanomas in the new 8th edition, American joint committee on cancer staging system? J Am Coll Surg. 2019;228:466-72.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant systemic therapy in high-risk melanoma. Melanoma Res. 2019;29:358-64.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of 3097 patients from the Japanese melanoma study database using the American joint committee on cancer eighth edition cancer staging system. In: J Dermatol Sci. Vol 94. 2019. p. :284-9.
- [CrossRef] [PubMed] [Google Scholar]
- Surviving cutaneous melanoma: A clinical review of follow-up practices, surveillance, and management of recurrence. Surg Clin North Am. 2014;94:989-1002.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous melanoma, Version 2, 2019 NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:367-402.
- [CrossRef] [PubMed] [Google Scholar]
- Update on adjuvant melanoma therapy. Curr Opin Oncol. 2018;30:118-24.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-60. quiz 761-4
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- [CrossRef] [PubMed] [Google Scholar]






