Translate this page into:
Neurological diseases and bullous pemphigoid: A case–control study in Iranian patients
2 Autoimmune Bullous Diseases Research Center, Tehran University of Medical Sciences, Tehran; School of Public Health, Gonabad University of Medical Sciences, Gonabad, Iran
Correspondence Address:
Hamidreza Mahmoudi
Autoimmune Bullous Diseases Research Center, Razi Hospital, Vahdate-Eslami Square, 11996 Tehran
Iran
| How to cite this article: Daneshpazhooh M, Khorassani J, Balighi K, Ghandi N, Mahmoudi H, Tohidinik H, Hamzelou S, Chams-Davatchi C. Neurological diseases and bullous pemphigoid: A case–control study in Iranian patients. Indian J Dermatol Venereol Leprol 2017;83:195-199 |
Abstract
Introduction: Neurological diseases are important co-morbidities found in association with bullous pemphigoid. Various neurological conditions (stroke, Parkinson's disease, dementia, epilepsy and multiple sclerosis) have been reported as associations of this bullous disease; whether these are significant has not been definitely proved. However, the presence of neurological conditions is a predictor of poorer prognosis.Objectives: Our aim was to examine the association of bullous pemphigoid and neurological diseases in Iranian bullous pemphigoid patients.
Methods: The medical records of one hundred and sixty consecutive bullous pemphigoid patients who presented to the Autoimmune Bullous Diseases Research Center, Tehran, Iran, from 2006 to 2011 were examined for evidence of any neurological disease. The control group comprised of 317 age- and sex-matched subjects.
Results: Neurological diseases were seen in 42 (26.4%) patients with bullous pemphigoid and in 29 (9.1%) controls (odds ratio: 3.53 (2.1–5.9), P< 0.001). Comparing cases to controls, stroke was seen in 17.5% versus 4.1%, odds ratio 4.96 (2.49–9.88); dementia in 5.6% versus 1.9%, odds ratio 3.09 (1.08–8.84); Parkinson's disease in 2.5% versus 2.2%, odds ratio 1.14 (0.33–3.94); epilepsy in 2.5% versus 0.6%, odds ratio 4.04 (0.73–22.3); and multiple sclerosis in 0 versus 0.3% odds ratio 1.00 (0.98–1.01).
Limitations: The main limitations of our study were referral bias, retrospective design and a rather low sample size.
Conclusions: Neurological diseases in general, and stroke and dementia in particular, were significantly associated with bullous pemphigoid in our study.
Introduction
Bullous pemphigoid is a chronic autoimmune subepidermal blistering disease of the skin characterized by the presence of circulating immunoglobulin G antibodies directed against two major hemidesmosomal components, the bullous pemphigoid antigen 230 and the bullous pemphigoid antigen 180 (bullous pemphigoid 180, BPAG2 or type XVII collagen).[1]
It is the most common autoimmune bullous disease in Western European countries. Its incidence varies from 6 to 7 people per million in Germany and France, to 30 per million in the UK.[1] Although the disease is rare in Asia in comparison with Europe, its prevalence is increasing in India, China and Iran.[1],[2] It is the second most common autoimmune bullous disease (11%) in Iran.[2]
Association of bullous pemphigoid with various neurological diseases such as multiple sclerosis, Parkinson's disease and dementia has been demonstrated in recent years. Bullous pemphigoid antigens or their isoforms have been identified in the brain and neuronal tissue in humans as well as in mice, and exposure of these hidden antigens through disruption of the blood-brain barrier, triggering an immune response and the production of cross-reactive autoantibodies, are possible mechanisms explaining this link.[3] However, this theory has not been confirmed in humans.
The presence of neurological disease in bullous pemphigoid patients has been shown to be associated with a poorer prognosis for bullous pemphigoid in recent studies.[4],[5] Our aim in this study was to investigate neurological diseases in Iranian patients with bullous pemphigoid, using a case-control method.
Methods
The files of bullous pemphigoid patients presenting to Autoimmune Bullous Diseases Research Center, Razi Hospital, Tehran, Iran, from 2006 to 2012 were examined. Razi Hospital is a tertiary referral dermatology hospital, affiliated to Tehran University of Medical Sciences. All patients with the following criteria were enrolled: Typical clinical findings of bullous pemphigoid, the presence of subepidermal bulla histopathologically and linear deposition of C3 and/or immunoglobulin G by direct immunofluorescence. In equivocal cases, direct immunofluorescence on salt-split skin was considered confirmatory.
For the control group, patients and their relatives presenting to tumor clinics of the hospital, relatives of patients attending the hospital wards of a general hospital affiliated to the same university (except the neurology ward), as well as people in neighborhood public places (parks) and the university were interviewed. These areas are covered by Razi Hospital for skin problems. Persons with no personal or family history of autoimmune bullous diseases were selected. The size of the control group was twice that of the case group. Controls were matched on age (P = 0.97) and gender (P = 0.97) with the cases. Data about neurological diseases was collected according to the past medical and drug history given by the patients or their relatives and recorded in their files. All the following neurological diseases were included: Stroke, dementia, Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis, spinal injuries and schizophrenia. The age of onset of the neurological disease and bullous pemphigoid was recorded as well. In the case of missing information, phone calls to patients or their family were used to complete or verify the data. The same data was collected for controls.
P value <0.05 was considered significant. SPSS version 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) was used for statistical analysis. Mean and standard deviation were used as quantitative parameters and number and percentage as qualitative variables. Comparisons among groups were performed using the χ2 test, Fisher's exact test and Student's t-test, whenever applicable. Crude odds ratio (95% confidence interval) was calculated for bivariate analysis. For variables with P < 0.2 logistic regression with enter method was performed and adjusted odds ratio (95% confidence interval) was calculated.
Results
One hundred and sixty patients fulfilled the criteria for bullous pemphigoid. Their mean age was 68.8 years (standard deviation = 18.6, range = 16–103). Eighty five (54%) patients were female and 75 (46%) were male. Forty two (26.3%) patients with bullous pemphigoid had at least one neurological disease. The mean age in patients with neurological disease was 81.2 years old (standard deviation = 8.3, range = 16–103) and greater than the mean age of those 118 patients without neurological disease, 64.4 years (standard deviation = 19.3, range = 17–99). The difference in mean age between patients with bullous pemphigoid with and without neurological disease was statistically significant (P < 0.001).
The mean age in the control group subjects with neurological diseases was 81.5 years (standard deviation = 11.2) which was greater than the mean age of control subjects without neurological disorders which was 66.1 years (standard deviation = 184.4, P < 0.001).
The female to male ratio of patients with neurological disease was 24/18 (1.3:1), while it was 61/57 (1.07:1) in the remaining patients. No significant gender differences were found between these groups (P = 0.52).
The frequency of neurological diseases was 9.1% (29/317) in the control group in comparison with 26.3% (42/160) in the case group. Bullous pemphigoid patients had significantly increased odds for neurologic diseases (odds ratio: 3.53; 95% confidence interval: 2.1–5.90, P < 0.001).
Among the 42 patients with bullous pemphigoid who had neurologic disease, the frequency of specific neurologic disorders was as follows: Stroke 28 (17.5%), dementia 9 (5.6%), Parkinson's disease 4 (2.5%) and epilepsy 4r (2.5%). None of the patients had multiple sclerosis. On the other hand, these figures for the control group were as follows: Stroke 13 (4.1%), dementia 6 (1.9%), Parkinson's disease 7 (2.2%), epilepsy 2 (0.6%) and multiple sclerosis 1 (0.3%).
The patients with bullous pemphigoid had significantly increased odds for stroke and dementia compared with controls (crude odds ratio for stroke, 4.96 [95% confidence interval: 2.49–9.88]; for dementia, 3.09 [95% confidence interval: 1.08–8.84], P < 0.001). Stroke, dementia and epilepsy were included in the multivariate analysis and the adjusted odds ratio was significant for stroke and dementia [Table - 1].
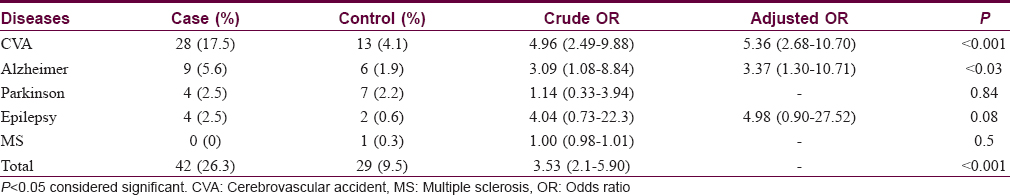
All the patients had developed bullous pemphigoid at least 1 year after the onset of neurologic disease.
Discussion
This case–control study was in agreement with previous worldwide studies confirming a link between neurological diseases and bullous pemphigoid. In addition, we did observe a significant association between bullous pemphigoid and stroke and dementia.
Twenty studies (excluding case reports) have been listed on PubMed reporting the association of neurological diseases and bullous pemphigoid till June 2015 [Table - 2]. Twelve were controlled studies, all of which reported increased odds for this association. The frequency of neurologic diseases varied from 23% to 55.8%, respectively.[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[16],[19]
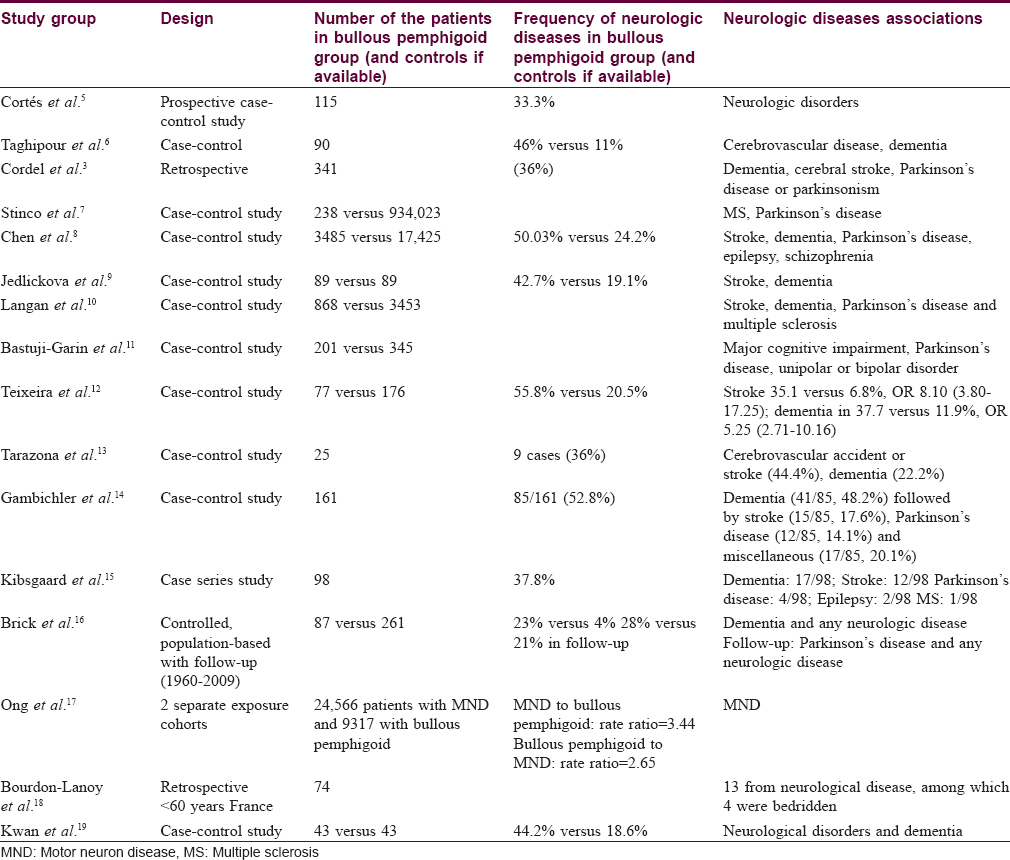
Various neurologic diseases have been reported in association with bullous pemphigoid including stroke, dementia, multiple sclerosis, epilepsy, Parkinson's disease, Shy–Drager syndrome and amyotrophic lateral sclerosis. When considering individual neurologic disorders, much variation is seen between different case–control studies. Cordel et al. found at least one neurological disorder in 123 of 341 patients with bullous pemphigoid in twenty French dermatology departments; dementia, cerebral stroke and Parkinson's disease or Parkinsonism accounted for nearly 90% of diseases.[3]
Bastuji-Garin et al. reported that bullous pemphigoid patients had higher rates of stroke, Parkinson's disease and unipolar or bipolar disorders.[11] Teixeira et al. found a significant association with cerebral stroke, dementia and Parkinson's disease in Portuguese patients.[12] Taghipour et al. found increased odds for cerebrovascular diseases and dementia in the UK.[6] Stinco et al., in an Italian study, showed an association with Parkinson's disease and multiple sclerosis by crossing the hospital discharge codes between bullous pemphigoid and other pathologies.[7] Chen found stroke, dementia, Parkinson's disease, epilepsy and schizophrenia in association with bullous pemphigoid in Taiwan.[8] In another study from the UK, Langan et al., observed significant associations for stroke, dementia, Parkinson's disease and multiple sclerosis when diagnoses up to 3 years before bullous pemphigoid were excluded.[10] Brick et al., in a population-based study in Minnesota, found that the odds of a previous diagnosis of any neurologic disorder or a history of dementia was significantly elevated among cases as compared with controls. Interestingly, both Parkinson's disease, specifically, and any type of neurologic disorder in general, were significantly more likely to develop during follow-up in patients with bullous pemphigoid than those without it.[16]
We found significantly increased associations of bullous pemphigoid with neurological diseases in general, and specifically with cerebrovascular accident and dementia. This is in accordance with the findings of Taghipour et al. and Jedlickova et al.[6],[9] Although the association with Parkinson's disease and epilepsy has been reported in many studies, our findings did not confirm it (crude odds ratio: 1.14 [95% confidence interval: 0.33–3.94]). A variety of factors, such as ethnicity, study design and control group size and selection may underlie this discrepancy.
According to our study, bullous pemphigoid patients with neurologic disease were significantly older than those without this comorbidity. The same was true for the control group. Previous investigators have reported variable results about this aspect. Our findings were in accordance with Cordel et al. and Gambichler et al. who found a difference of 4.5 and 7 years between the mean age of patients with and without neurologic disease, respectively.[3],[14] In contrast, there was no age difference in the study by Taghipour et al.[6] Interestingly, Taghipour et al. found a younger age at onset for bullous pemphigoid in patients with multiple sclerosis in a different study.
Neurological disease in bullous pemphigoid adversely affects not only the quality of life but also the prognosis (mortality [5] and relapse [4]) of these elderly patients. More recently, the role of comorbidities, including neurologic disorders, in the prognosis of bullous pemphigoid have been investigated. Interestingly, Fichel et al. found dementia as an independent factor associated with bullous pemphigoid relapse during the first year of treatment [4] while Cortés et al. showed that the presence of neurological diseases at diagnosis was associated with increased mortality.[20] According to Cai et al., only Parkinson's disease remained significantly associated with elevated mortality in the multivariate analysis.[21] Lee et al. also found old age, stroke, diabetes and delayed diagnosis increase the risk of mortality in patients with bullous pemphigoid.[22]
Pathogenically, the significance of the association of bullous pemphigoid and neurologic disease remains speculative. Bullous pemphigoid antigens or their isoforms have been identified in brain and neuronal tissue as well as the skin. The presence of similar hemidesmosomal protein antigens (especially bullous pemphigoid 230) in skin and neurons may be important factors in the pathogenesis of bullous pemphigoid-associated neurological disorders. It has been hypothesized that neurological diseases may expose antigens such as bullous pemphigoid 180 and bullous pemphigoid 230 to the immune system and trigger a subsequent immune response that may result in the manifestation of bullous pemphigoid. This hypothesis is supported by the fact that the neurologic manifestations usually occurs several years before the onset of bullous pemphigoid.[14]
According to Chen et al.[23] a majority of serum samples from patients with bullous pemphigoid and neurologic disease were found to recognize a 230-kD protein of human brain extract.[23] Some studies have demonstrated that both human skin and brain contain bullous pemphigoid 180 antigen, and normal brain and retina have bullous pemphigoid 230 antigen.[23],[24],[25] Gambichler et al. showed recently that raised bullous pemphigoid 180 antigen titers and blood eosinophil are independent predictors for the presence of neurologic disorders in bullous pemphigoid patients, but unlike previous studies, they could not detect bullous pemphigoid 180-specific immunofluorescence in an animal model.[14],[23]
The major limitation of our study may be a referral bias, since the study was undertaken in a major tertiary center. Low sample size, as well as the retrospective design, may be other drawbacks, which may have lead to non-significant findings for some neurologic diseases.
Conclusions
According to our study, neurological diseases in general, as well as stroke and dementia, were significantly associated with bullous pemphigoid. However, this association was not significant for other individual neurological diseases. Further prospective studies are suggested to evaluate the impact of neurological diseases on the prognosis of bullous pemphigoid.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: Pemphigus and bullous pemphigoid. Arch Dermatol Res 2015;307:291-8.
[Google Scholar]
|
| 2. |
Daneshpazhooh M, Chams-Davatchi C, Payandemehr P, Nassiri S, Valikhani M, Safai-Naraghi Z. Spectrum of autoimmune bullous diseases in Iran: A 10-year review. Int J Dermatol 2012;51:35-41.
[Google Scholar]
|
| 3. |
Cordel N, Chosidow O, Hellot MF, Delaporte E, Lok C, Vaillant L, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology 2007;215:187-91.
[Google Scholar]
|
| 4. |
Fichel F, Barbe C, Joly P, Bedane C, Vabres P, Truchetet F, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: A multicenter, prospective study. JAMA Dermatol 2014;150:25-33.
[Google Scholar]
|
| 5. |
Cortés B, Marazza G, Naldi L, Combescure C, Borradori L; Autoimmune Bullous Disease Swiss Study Group. Mortality of bullous pemphigoid in Switzerland: A prospective study. Br J Dermatol 2011;165:368-74.
[Google Scholar]
|
| 6. |
Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: A case-control study. Arch Dermatol 2010;146:1251-4.
[Google Scholar]
|
| 7. |
Stinco G, Codutti R, Scarbolo M, Valent F, Patrone P. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol 2005;85:136-9.
[Google Scholar]
|
| 8. |
Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br J Dermatol 2011;165:593-9.
[Google Scholar]
|
| 9. |
Jedlickova H, Hlubinka M, Pavlik T, Semradova V, Budinska E, Vlasin Z. Bullous pemphigoid and internal diseases – A case-control study. Eur J Dermatol 2010;20:96-101.
[Google Scholar]
|
| 10. |
Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: A population-based case-control study. J Invest Dermatol 2011;131:631-6.
[Google Scholar]
|
| 11. |
Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: A prospective case-control study. J Invest Dermatol 2011;131:637-43.
[Google Scholar]
|
| 12. |
Teixeira VB, Cabral R, Brites MM, Vieira R, Figueiredo A. Bullous pemphigoid and comorbidities: A case-control study in Portuguese patients. An Bras Dermatol 2014;89:274-8.
[Google Scholar]
|
| 13. |
Tarazona MJ, Mota AN, Gripp AC, Unterstell N, Bressan AL. Bullous pemphigoid and neurological disease: Statistics from a dermatology service. An Bras Dermatol 2015;90:280-2.
[Google Scholar]
|
| 14. |
Gambichler T, Segert H, Höxtermann S, Schmitz L, Altmeyer P, Teegen B. Neurological disorders in patients with bullous pemphigoid: Clinical and experimental investigations. J Eur Acad Dermatol Venereol 2015;29:1758-62.
[Google Scholar]
|
| 15. |
Kibsgaard L, Bay B, Deleuran M, Vestergaard C. A retrospective consecutive case-series study on the effect of systemic treatment, length of admission time, and co-morbidities in 98 bullous pemphigoid patients admitted to a tertiary centre. Acta Derm Venereol 2015;95:307-11.
[Google Scholar]
|
| 16. |
Brick KE, Weaver CH, Savica R, Lohse CM, Pittelkow MR, Boeve BF, et al. A population-based study of the association between bullous pemphigoid and neurologic disorders. J Am Acad Dermatol 2014;71:1191-7.
[Google Scholar]
|
| 17. |
Ong EL, Goldacre R, Taghipour K. The relationship between motor neuron disease and bullous pemphigoid: An English cohort study. J Am Acad Dermatol 2013;69:836-7.
[Google Scholar]
|
| 18. |
Bourdon-Lanoy E, Roujeau JC, Joly P, Guillaume JC, Bernard P, Prost C, et al. Bullous pemphigoid in young patients: A retrospective study of 74 cases. Ann Dermatol Venereol 2005;132:115-22.
[Google Scholar]
|
| 19. |
Kwan Z, Lai YN, Ch'ng CC, Tan AH, Tan LL, Robinson S, et al. The association between bullous pemphigoid and neurological disorders in a selected Malaysian population. Med J Malaysia 2015;70:81-5.
[Google Scholar]
|
| 20. |
Cortés B, Khelifa E, Clivaz L, Cazzaniga S, Saurat JH, Naldi L, et al. Mortality rate in bullous pemphigoid: A retrospective monocentric cohort study. Dermatology 2012;225:320-5.
[Google Scholar]
|
| 21. |
Cai SC, Allen JC, Lim YL, Chua SH, Tan SH, Tang MB. Mortality of bullous pemphigoid in Singapore: Risk factors and causes of death in 359 patients seen at the National Skin Centre. Br J Dermatol 2014;170:1319-26.
[Google Scholar]
|
| 22. |
Lee JH, Kim SC. Mortality of patients with bullous pemphigoid in Korea. J Am Acad Dermatol 2014;71:676-83.
[Google Scholar]
|
| 23. |
Li L, Chen J, Wang B, Yao Y, Zuo Y. Sera from patients with bullous pemphigoid (BP) associated with neurological diseases recognized BP antigen 1 in the skin and brain. Br J Dermatol 2009;160:1343-5.
[Google Scholar]
|
| 24. |
Claudepierre T, Manglapus MK, Marengi N, Radner S, Champliaud MF, Tasanen K, et al. Collagen XVII and BPAG1 expression in the retina: evidence for an anchoring complex in the central nervous system. J Comp Neurol 2005;487:190-203.
[Google Scholar]
|
| 25. |
Seppänen A, Autio-Harmainen H, Alafuzoff I, Särkioja T, Veijola J, Hurskainen T, et al. Collagen XVII is expressed in human CNS neurons. Matrix Biol 2006;25:185-8.
[Google Scholar]
|
Fulltext Views
4,332
PDF downloads
1,970





