Translate this page into:
New insights in the pathogenesis of type 1 and type 2 lepra reaction
Correspondence Address:
Deepika Pandhi
Department of Dermatology and Sexually Transmitted Disease, University College of Medical Sciences, Guru Teg Bahadur Hospital, University of Delhi, Delhi - 110-095
India
| How to cite this article: Pandhi D, Chhabra N. New insights in the pathogenesis of type 1 and type 2 lepra reaction. Indian J Dermatol Venereol Leprol 2013;79:739-749 |
Abstract
In the current scenario of leprosy elimination, lepra reactions (LRs) remain a major persistent problem. Type 1 LR (T1LR) and type 2 LR (T2LR) are the major causes of nerve damage and permanent disabilities. The immunopathogenesis of LR have recently become an important field of research, since it may provide the relevant targets for the early detection and control of these episodes. Presently, there are no uniformly acceptable laboratory markers for LR. Genetic and serum markers in human host may predict susceptibility to reactions as well as progression of nerve damage in leprosy. Therefore, a deeper understanding of the molecular mechanisms involved in LR may provide a rational strategy for early diagnosis and prevention of the catastrophic consequences of LR.Introduction
A major problem in the management of leprosy patients is the occurrence of "reactions." These reactions are the consequences of the dynamic nature of the immune response to Mycobacterium leprae (M. leprae) that may occur before, during, or following the completion of multi-drug therapy (MDT). There are two major types of lepra reactions (LR). Type 1 LR (T1LR), also described as "reversal" reaction, is a type IV hypersensitivity reaction, that occurs in borderline leprosy patients with cellular immune responses to M. leprae antigenic determinants, [1] and is characterized by acute inflammation of pre-existing skin lesions or by the appearance of new lesions and/or neuritis. [2] Approximately 95% of T1LR cases are diagnosed simultaneously with leprosy or during the first 2 years of MDT. [3] Erythema nodosum leprosum (ENL), the most common manifestation of type 2 LR (T2LR), is an immune complex mediated complication of lepromatous leprosy (LL). T2LR presents with skin lesions (red, painful, and tender subcutaneous lesions), fever, and systemic inflammation that may affect the nerves, eyes, joints, testes, and lymph nodes. Most of the T2LRs occur during the first year of MDT. [4]
Reactions are responsible for most of the permanent nerve damage, deformity, and disability. [1],[5] Clinically detectable nerve function impairment (NFI) occurs in approximately 10% of paucibacillary and 40% of multibacillary leprosy patients, particularly in patients with T1LR. [6] It has, however, been suggested that "silent neuropathy" due to sub-clinical neural involvement may take place in virtually all leprosy patients and that 30% of the nerve fibres need to be destroyed before sensory impairment becomes detectable. [7]
Epidemiology
The prevalence rate of T1LR has been reported to vary from 8.9 to 35.7% in various prospective and retrospective studies. [8],[9] The prevalence of ENL reactions in BL and LL cases has wide geographic variation; varying from 19-26% in Asia to 37% in Brazil. [8]
Need To Know the Pathogenesis of Leprosy Reactions
Many years after elimination of leprosy has been achieved, the occurrence of reactions in leprosy patients continues to be a formidable challenge mainly owing to its role in causing nerve damage and disability. Because LR may occur months or even years after MDT completion, related disabilities are expected to continue to occur even under the unlikely scenario of leprosy eradication. Cohort studies estimate that disability in leprosy ranges from 16% to 56%, mainly attributable to the occurrence of reactional episodes. Even with adequate treatment, 40% of patients with T1LR may present with permanent nerve damage. [10] A recent study by van Brakel et al., using nerve conduction studies and quantitative sensory testing, has demonstrated that individuals experiencing neuritis, NFI, or reactional episodes, either alone or in combination, have evidence of subclinical neuropathy up to 12 weeks prior to clinically detectable changes. [11] This indicates that there is a potential for early diagnosis and intervention for prevention of clinically apparent nerve damage and deformity. In this context, it is pertinent to identify reliable laboratory tests to aid in the early diagnosis of leprosy reactions to monitor efficacy of treatment.
Treatment of LR is mostly instituted following a clinical diagnosis. Histological features pathognomonic of T1LR are not adequately standardized. [12] This fact along with inter-observer variation in histological diagnosis account for delayed diagnosis of LR in a large percentage of cases. Data from a recent clinico-pathological study showed that the clinical diagnosis of T1LR is accompanied by recognisable histological changes in only 60% of cases. In the above mentioned study, an attempt was made to increase the frequency of diagnosis and reduce the inter-observer variations by establishing five key variables for diagnosing T1LR: Dermal oedema, intra-granuloma oedema, giant cell size, giant cell numbers, and HLA DR expression. [12] Evaluation of these may aid in predicting patients likely to progress to clinically apparent T1LR.
Hence, there is an urgent need to understand the immuno pathogenesis and identify the cytokine profiles associated with these reactions, to provide predictive and prognostic biomarkers for early identification of patients who are at an increased risk of LR, for eventual monitoring of treatment efficacy and to devise novel treatments to reduce nerve damage.
Agent Virulence Factors
M. leprae antigenic determinants have been demonstrated in the nerves and skin of patients experiencing T1LR. The antigens were localised to Schwann cells and macrophages. [13] A study of Brazilian patients with slit-skin smear negative, single lesion, and paucibacillary leprosy concluded that individuals with M. leprae DNA detectable by PCR in the skin lesion were more likely to experience a T1LR than those in whom M. leprae DNA was undetectable. [14] Several M. leprae-specific genes have been used as targets in the diagnosis and treatment of leprosy. Genetic analysis of accA3, a metabolism-associated protein revealed higher expression levels of this gene in biopsy specimens of reaction cases compared with control patients of same clinical type without clinically evident reaction. The authors have indicated its usefulness as a potential marker for monitoring reactions. [15] DNA and mRNA of mycobacterial hsp18 gene have been analyzed to look for the role of viable bacilli in LR. The study concluded that a significant amount of mRNA for the hsp18 gene was present in T1LR. [16] These findings point towards considering the need for reinstituting MDT to eliminate the residual pathogen in reaction cases, which could be a better approach in conjunction with anti-inflammatory agents in controlling late reactions and relapses. Few studies have also implicated a hypothesis of antigenic triggers in T1LR, leading to expansion of both cross-reactive and specific T-cells. The role of infection by mycobacteria other than M. leprae as a trigger in T1LR was suggested by an increased risk of reactions in patients vaccinated with Mycobacterium w.[17] In an interesting case report of a patient with T1LR, an increase in T-cell reactivity to a peptide from the 38 kDa antigen of M. tuberculosis, whose expression is restricted to M. intracellulare and M. tuberculosis complex, was documented. [18]
Host Related Risk Factors
Various host-related factors have been reported as risk factors for T1LR; these include increasing age, extensive disease, and having a positive slit-skin smear. [14],[19],[20] Household contacts were also a significant predictor for LR in females from an endemic area of Brazil, suggesting that leprosy reactions may be triggered by an external spreading of M. leprae by healthy carrier family members. [21] Furthermore, individuals who present with WHO disability grades 1 and 2 at the time of diagnosis were significantly more likely to have severe T1LR. [22] Concurrent infection could also be an exacerbating factor in LR. In a recent study from Brazil, patients with oral infections and reactional episodes had higher level of serum CRP and interferon-gamma-induced protein (IP-10) than those with LR without oral infections. [23]
LL and a bacillary index greater than 4+ are established risk factors for T2LR. Intercurrent infections, vaccination, stress pregnancy, lactation, and puberty have also been implicated in its causation, but these associations need to be validated in prospective studies. [24]
Host-Related Immunological Factors
Surprisingly, little genotypic variation exists between strains of M. leprae, a fact inconsistent with the high degree of variability in virulence and disease penetrance between individuals. This suggests that success of infection and leprosy progression rests in large part upon the host′s immune response and genetic complement. More than 99% of the population is believed to develop adequate protective immunity to infection and does not develop clinically detectable symptoms. [25] The intracellular mechanisms leading to mycobacteria-induced cytokine response are not yet fully characterized. However, many authors have focussed on the present hypothetical pathomechanism and tried to look for the relevant immune cells and cytokines in serum as well as tissues affected by M. leprae.
Innate Immunity
The ability of the host to rapidly detect invading pathogens is an important feature of the innate immune system and is mediated in part by pattern recognition receptors that recognize various classes of microbial ligands. In particular, Toll-like receptor 2 (TLR2) has been shown to be involved in the recognition of mycobacterial lipoproteins. TLR stimulation also activates the nuclear transcription factor NF-kB, which modulates the transcription of many immune response genes. [26]
Human Polymorphism as Clinical Predictor of Leprosy Reactions
TLR gene polymorphisms appear to affect the risk of acquiring leprosy and T1LR probably due to the stronger immune response to bacterial antigens. In a cohort of Ethiopian patients, a single nucleotide polymorphism (SNP) in TLR2 (597C > T) was associated with protection against T1LR, and a 280-bp microsatellite marker was associated with an increased risk of T1LR, whereas the TLR4 SNP (1530G > T) was more frequent in individuals with T1LR. [27],[28] Similarly, TLR2 and TLR4 were found to be associated with T1LR in a study comprising of 21 Nepalese patients. Their role was further elucidated by reduction in the gene and protein expression of TLR2 and TLR4 in these patients during corticosteroid treatment. [29] In another cohort of 238 Nepalese patients, non-synonymous SNP rs5743618 (I602S) of TLR1 was found to be protective against T1LR. [30] Another non-synonymous polymorphism of TLR1 (N248S) was associated with T2LR, with the N alleles being more frequent among patients with T2LR. [31] The role of TLR in LR can be implicated in achieving treatment strategies. TLR agonists as therapeutic agents might be evaluated in LR to generate pro-inflammatory responses without tissue injury. On the other hand, TLR antagonists could be useful in preventing immunopathological manifestations of the innate immune response to M. leprae infection.
Variants of HLA genes, HLA-DR B1 in particular, have also been associated with leprosy; both protective and risk alleles have been described. [32] In LR, HLA-DR expression is a characteristic feature and has been established as one of the key marker in biopsy. [12]
Other Genetic Factors
Interleukin (IL)-6 promotes cell-mediated immune reactions, notably by stimulating IL17, and by inhibiting regulatory T cells. IL-6 is also considered as a key player in acute-phase reaction, which is one of the earliest responses to insults. Significant association between T2LR and IL-6 tag SNPs was found implicating IL-6 in the pathogenesis of T2LR and indicate this cytokine as a possible valuable predictive marker. [33] Ethnic background may play an important role in the frequency of the above mentioned gene polymorphisms and, thus, further work is warranted to clarify the role of these in the development of reactions.
Adaptive Immunity
Activation of innate immunity leads to cytokine production and the expression of co-stimulatory molecules that result in activation of adaptive immune system cells. T1R is due to an increase in cell-mediated response to the M. leprae antigenic determinants characterized by activity of T helper (Th)-1 lymphocytes expressing IL-2 and IFN-γ. [10],[34] IL-12 is consistently expressed and IL-4 is absent. [35] The IFN-γ and TNF-α producing CD4 cells and T cytotoxic cells are selectively increased with clearing of bacilli and concomitant tissue damage. [36]
In contrast with T1LR, a predominant Th2 cytokine profile has been observed in T2LR with increased expression of IL-6, IL-8, and IL-10 as well as sustained production of Th2 cytokines, IL-4, and IL-5. [37] T2LR is a systemic inflammatory response characterized by neutrophil infiltration, activation of complement, extra-vascular immune complexes, and high levels of TNF-α in tissue lesions and circulation. [38] Major aspects of this pathway include the following: (i) FcR or TLR2 induction of IL-1b release; (ii) endothelial activation, including the upregulation of E-selectin and subsequent neutrophil binding; and (iii) upregulation of inflammatory mediators associated with both neutrophils and monocytes/macrophages. [39] Thalidomide targets several individual events in the inflammatory pathway reducing neutrophil infiltration in lesions. [40]
The IL-17F producing Th17 cells have been identified as a new subset of the T helper cells and as potential mediators of inflammation associated with various autoimmune and mycobacterial diseases. [41] Recent studies have revealed that Th17 cells maybe involved in the immunopathogenesis of T2LR, and IL17F gene expression was upregulated before and after thalidomide treatment. [42] Mycobacteria and their cell wall components, such as LAM, have been reported to induce NF-κB nuclear translocation and MAP kinase activation, both being important events for cytokine production and cell activation. [43] Thalidomide has also been found to suppress NF-κB transcription, DNA binding activity, and activation-induced by M. leprae antigenic determinants in primary human cells that consequently results in reduced cytokine production and clinical resolution of T2LR. [44]
In a cohort of 61 patients, including six cases each of T1LR and ENL, rise in IL-1β and IFN-γ was said to predict development of both reactional episodes; whereas, increment in TNF-α and IL-10 occurred in T1LR and ENL, respectively. [45]
Implications in Diagnosis: Markers of Leprosy Reactions in Skin and Nerve
The pro-inflammatory cytokine TNF-α is crucial to anti-mycobacterial immunity and plays an important role in granuloma formation during mycobacterial infection. [46] TNF-α protein has been detected in biopsies taken from leprosy patients with skin reactional lesions of both T1LR and ENL. [37],[46],[47] Inducible Nitric Oxide Synthase (iNOS) is an enzyme responsible for synthesis of reactive nitrogen radicals involved in killing of mycobacteria. [39] High levels of iNOS have been detected in skin biopsies from Indian and Ethiopian leprosy patients experiencing T1LR. [48] One of the recent prospective study from a tertiary hospital in North India detected high levels of TNF-α, transforming growth factor (TGF)-β, and iNOS by immunohistochemistry in biopsies from patients with T1LR and iNOS in the biopsies with ENL. The authors concluded that these cytokines were significantly associated with leprosy skin and nerve reactions and may be of use in the diagnosis and assessment of difficult reactional lesions. [49]
The tissue expression and the role of cyclooxygenase (COX) and vascular endothelial growth factor (VEGF) have also been postulated in the pathogenesis of leprosy and T1LR. VEGF and the endothelial cell receptor KDR, were over expressed in the granuloma cells, vascular endothelium, and overlying epidermis in T1LR, in comparison with non-reactional leprosy. [50] COX2 was found to be consistently expressed in cells of the mononuclear-macrophage lineage across the leprosy spectrum. In addition, T1LR lesions showed COX2 expression in microvessels, nerve bundles, and isolated nerve fibres. The same sites also showed expression of VEGF. [51] VEGF enhances prostaglandin production through COX2 stimulation and prostaglandin synthase expression. This causes vascular changes leading to tissue edema, which is characteristic of T1LR. With progression of T1LR, edema occurring in nerve fibres and bundles may lead to permanent nerve damage, which is the most important long-term sequela of T1LR. [51] These considerations suggest that selective COX2 inhibitors, which are currently used in several inflammatory conditions, could be considered for T1LR treatment, particularly at its early stage, to reduce acute symptoms and possibly prevent long-term nerve damage. Furthermore, they could be useful to prevent T1LR recurrence in unstable forms of the disease.
The CC chemokines, regulated upon activation, normal T cell expressed and secreted (RANTES) and monocyte chemoattractant protein 1 (MCP-1), predominantly attracts monocytes and lymphocytes. MCP-1 and RANTES were elevated in skin lesions of T1LR as compared to non-reactional leprosy, suggesting a role for these chemokines in migration and activation of the monocytes and T-lymphocytes in T1LR. [52]
CXC ligand 10 (CXCL 10) is a chemokine induced primarily by IFN-γ, produced constitutively by macrophages, T cells, and keratinocytes, which promote chemotaxis of T cells to sites of tissue inflammation. [53] CXCL 10 mRNA levels in skin biopsy of patients with T1LR was found to be elevated compared to biopsy specimens from the same patients prior to the reaction, [54] probably attract Th1 type cells to the reactional inflammatory sites in the skin.
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes responsible for extracellular matrix (ECM) remodelling and the regulation of the trans-ECM migration of leukocytes, an important step in inflammatory processes as well as in infectious diseases. These enzymes can be produced by several skin cells such as keratinocytes, langerhans cells, and dermal fibroblasts. MMP mRNA expression levels was found to be increased in skin biopsy of LR (especially T2LR) correlating with the expression of IFN-γ and TNF-α in these biopsies. [55] Hence, MMPs may also be implicated in the local and systemic responses to M. leprae infection, which may open new opportunities for therapeutic interventions.
Implications in Diagnosis: Serological Markers of Leprosy Reactions
Circulating profiles of the cytokines involved in immunopathogenesis may act as potential plasma markers to identify the disease early and predict the occurrence of LR. Literature provides strong evidence for the association of IL-6 with leprosy and its reactional states. A recent analysis of 27 plasma factors, including cytokines, chemokines, and growth factors, revealed IL-6 as the only biomarker of both T1LR and T2LR when compared with leprosy-affected individuals without reaction. [56]
Studies have shown elevated serum level of TNF-α, IL-2R in T1LR. [57],[58] Macrophage activation plays an important role in the control of M. leprae infection. A macrophage activation marker, neopterin, has also been shown to be useful in the diagnosis of T1LR as well as in monitoring response to steroid treatment. [58]
Along with increased tissue expression of CXCL 10 as mentioned above, its plasma levels has also been observed to be elevated in association with T1LR. [56] This association was further confirmed by another study, which showed strong association between circulating CXCL10 and the occurrence of T1LR. [54] Similarly, in addition to increased tissue expression of the protein MMP in biopsies from LRs, increased serum levels of MMP-9 were also detected in patients with LR versus controls. [55]
Chemokine ligand 11 (CCL11), a chemokine induced by IFN-γ, produced by monocytes, has also been identified as a potential plasma marker of T2LR. CCL11 is a potent chemo attractant for eosinophils and Th2 lymphocytes, to inflammatory sites. [39]
The INFIR cohort study from North India confirmed the previously proposed association between PGL-1 antibody levels and the occurrence of reactions and nerve damage. [59] Serum circulatory levels of the recently identified cytokine, IL-17F, are elevated during T1LR in the borderline spectrum of the disease. [60] IL7 is a key regulator of B cell development and proliferation and is essential for the survival of naοve and memory T cells, especially CD4 memory cells. [61],[62] Elevated circulating levels of IL7 were detected in T2LR, supporting a role for both B-cell and T-cell mediated mechanisms in this reaction. [56] One of the acute-phase protein alpha-1-acid glycoprotein (AGP) level was found to be higher in untreated ENL cases as compared with LL. Treatment with thalidomide has been shown to reduce the levels of AGP to normal. [63]
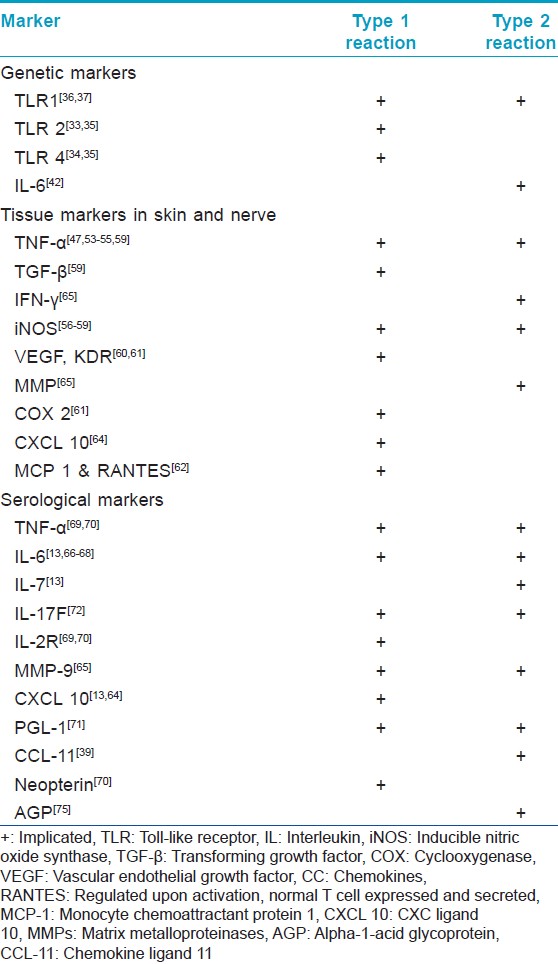
The relevant genetic polymorphisms and markers of LR in tissue and serum are summarized in [Table - 1].
Immunopathogenesis of Nerve Damage
The major complication of T1LR in leprosy is peripheral nerve damage. Human Schwann cells may be the central players in leprosy nerve damage. The destruction of Schwann cells is likely as a result of collateral damage as non-specific bystander effects during inflammation mediated mainly by TNF-α and also from the direct effect of CD4+ cytolytic T cells. TNF-α hardly has a toxic effect on Schwann cells on its own, but in combination with TGF-β, it has been reported to cause significant Schwann cell detachment and lysis. [64] Another likely immunopathogenic mechanism of Schwann cell and nerve damage in leprosy is that infected Schwann cells process and present antigens of M. leprae to antigen-specific, inflammatory type 1 T cells and that these T cells subsequently damage and lyse infected Schwann cells. Although this process can involve both CD8 and CD4 cytotoxic T cells, particularly, the latter type may be of importance because CD4 + T cells are present in higher numbers in the centre of granulomas of leprosy patients with T1LR. [65]
The role of innate immune response in nerve injury in leprosy has also been investigated. Human Schwann cells also express TLR2 and TLR2-positive Schwann cells in leprosy lesions undergo apoptosis, potentially contributing to nerve damage in leprosy. [66] Nerve damage can also occur in the absence of apoptosis or lysis because of demyelination upon exposure to M. leprae in the absence of immune cells. [67] [Figure - 1] summarizes the pathways implicated in destruction of Schwann cell, leading to nerve damage in leprosy.
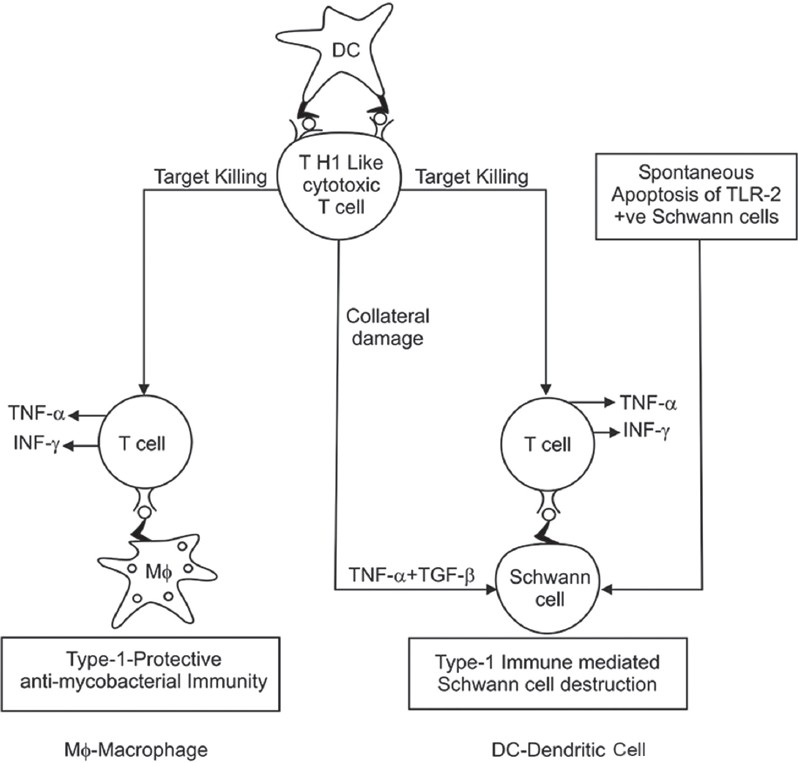 |
| Figure 1: Mechanism of Schwann cell damage |
Markers of Nerve Damage
Few studies have looked at laboratory parameters as risk factors for impending nerve damage. A change in TNF-α levels rather than the absolute level prior to an event was predictive of a new NFI. [68] PGL1 is involved in the M. leprae invasion of Schwann cells through the basal lamina in a laminin-2-dependent pathway. [69] The INFIR cohort study from North India confirmed the association between PGL-1 antibody levels and the occurrence of nerve damage. [68] Thus, apart from the clinical risk factors including multibacillary leprosy and the presence of existing nerve damage at the time of diagnosis, [22] serological parameters might be a useful indicator for nerve damage and should be further evaluated for this purpose.
Role of Cortisol-Cortisone Shuttle
LR may be precipitated by a breakdown of the mechanisms that normally regulate the effective concentration of endogenous glucocorticoids (cortisol) in the skin. The concentration of cortisol in a tissue is regulated by a reversible enzyme "shuttle" that can deactivate cortisol by converting it to cortisone or activate cortisone by converting it to cortisol. The activity of this shuttle and the direction in which it operates is regulated by numerous factors including cytokines. This results in large swings in the effective cortisol concentration at sites of inflammation at different phases of an inflammatory response. It has been suggested that changes in the activity of the shuttle in leprosy lesions may predispose to reactions, requiring exogenous steroid supplements to regain control of the inflammation. [70] Gene expression of 11beta-hydroxysteroid dehydrogenase type 2, which converts cortisol to cortisone, was found to be downregulated in the skin from T1LR lesions and showed upregulation after prednisolone treatment. [71] Thus, the cortisol-cortisone shuttle might be a potential target for newer therapeutic options in future.
Role of Apoptosis
One of the hypotheses in pathogenesis of LR is the induction of programmed cell death in macrophages due to M. leprae antigenic determinants leading to reduction in bacterial load. The evidence in favor of this hypothesis was supported by an in vitro study, which also found an enhanced rate of spontaneous apoptosis in LR as compared to lepromatous patients without evident reactions. [72] Furthermore, apoptosis studied by histopathology, DNA fragmentation and electrophoresis was more common in T2LR patients as compared to those without reaction. [73] Thus, the enhanced apoptosis seems to be a contributing factor to tissue damage in LR.
Leprosy Reactions and Hiv
The most interesting phenomenon associated with the interaction between HIV and leprosy infection is the higher incidence of T1LR, suggesting that the immune regulation of each disease is independent. In one study, CD38 antigen, a cellular activation marker previously associated with HIV pathogenesis, [74] was found to be significantly elevated in the CD8+ T cells of T1LR individuals and diminished after prednisone therapy. [75] Thus, CD38 expression in CD8+ T cells may be an interesting tool for identifying HIV/leprosy individuals at risk for T1LR. However, caution is required as CD38 expression also predicts viral replication, progression of HIV to AIDS, and failure of HAART. [76] There is paucity of data on the effect of HIV infection on the frequency or clinical presentation of T2LR in co-infected patients.
Implications in Treatment
Corticosteroids are the drugs of choice in the treatment of T1LR due to their inhibition of the pro-inflammatory cytokine milieu that aid in the recovery of NFI. The current WHO Global Strategy document recommends treatment of severe T1LR with "a course of steroids, usually lasting 3-6 months," which is often inadequate. [77] The recent Cochrane systematic review of "Corticosteroids for treating nerve damage in leprosy" identified only three randomised controlled trials (RCT) that met the review criteria, and it concluded that long-term steroids did not have significant effect on the outcome of nerve damage and that further RCTs are required to identify the best treatment regimen of steroids in the management of severe reactions and NFI. [78] An RCT comparing different steroid regimes for the management of severe T1LR suggested that duration rather than dose of treatment with prednisolone may be more important in controlling T1LR. [79] Azathioprine in combination with a short course of prednisolone has been reported to be as effective as a 12-week course of prednisolone in the management of T1LR in 40 patients. [80] Ciclosporin has also been used with some success. [81]
The main aims in the management of T2LR are the control of inflammation, pain relief, and prevention of further episodes. [82] Mild cases of T2LR can be treated with non steroidal anti-inflammatory drugs (NSAIDs). Prednisolone is commonly used for the management of moderate to severe ENL. Thalidomide is another drug effective in moderate to severe T2LR. Its beneficial effect is primarily thought to be due to its action on TNF, but other mechanisms may also play a part. [39],[83] Few of the recently implicated influence on host immunity mechanisms have been discussed above. Favourable response to colchicine, azathioprine, [84] methotrexate, [85] oral zinc, [86] and the chimeric anti-TNF monoclonal antibody, infliximab, [87] has been reported in ENL.
Treatment of the LR causes clinical improvement, but changes in the inflammatory cytokines considerably lag behind and, in some, may remain unchanged. [88] Furthermore, due to the controversies about the optimum type of treatment for LR, laboratory tests for monitoring the disease activity will be of considerable value for clinicians and leprosy control programs.
Future Directions
These data implicate the role of certain cytokines released on account of altered immune response as a result of genetic polymorphism and presence of individual risk factors in a leprosy patient who develops LR. However, a limitation of serum cytokine measurement in association with leprosy is that most studies measured one or few cytokines or cellular activation markers and/or included small number of subjects. Moreover, contradictory results with respect to the predominant cytokines have also been reported, which may be attributed to different assay techniques and populations examined and the presence of confounding factors as many of these pro-inflammatory markers are not specific to leprosy.
However, these results pave the way towards the application of new therapeutic interventions for LR. Studies with larger numbers of patients could attempt to elucidate the role of these cytokine markers in LR by examining their serum levels and expression in the skin lesions of these patients prior to the onset of reaction and comparing it with changes at the onset of reaction and during treatment. Further studies are recommended to see the effect of prophylactic therapy with anti-inflammatory drugs on prevention of development of overt or silent neuritis during antimicrobial treatment. Addressing these questions in future could also help in the prevention of nerve damage induced sequelae leading to deformities and disabilities, which are the hallmark of leprosy.
Acknowledgement
We would like to acknowledge Mrs Sunita Samvedi for executing the illustration.
| 1. |
Scollard DM, Smith T, Bhoopat L, Theetranont C, Rangdaeng S, Morens DM. Epidemiologic characteristics of leprosy reactions. Int J Lepr Other Mycobact Dis 1994;62:559-67.
[Google Scholar]
|
| 2. |
Van Brakel WH, Nicholls PG, Das L, Barkataki P, Suneetha SK, Jadhav RS, et al. The INFIR Cohort Study: Investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in north India. Lepr Rev 2005;76:14-34.
[Google Scholar]
|
| 3. |
Ranque B, Nguyen VT, Vu HT, Nguyen TH, Nguyen NB, Pham XK, et al. Age is an important risk factor for onset and sequelae of reversal reactions in Vietnamese patients with leprosy. Clin Infect Dis 2007;44:33-40.
[Google Scholar]
|
| 4. |
Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, et al. Clinical course of erythema nodosum leprosum: An 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg 2006;74:868-79.
[Google Scholar]
|
| 5. |
Naafs B. Leprosy reactions. New knowledge. Trop Geogr Med 1994;46:80-4.
[Google Scholar]
|
| 6. |
Richardus JH, Finlay KM, Croft RP, Smith WC. Nerve function impairment in leprosy at diagnosis and at completion of MDT: A retrospective cohort study of 786 patients in Bangladesh. Lepr Rev 1996;67:297-305.
[Google Scholar]
|
| 7. |
Pearson JM, Ross WF. Nerve involvement in leprosy: Pathology, differential diagnosis and principles of management. Lepr Rev 1975;46:199-212.
[Google Scholar]
|
| 8. |
Kahawita IP, Walker SL, Lockwood DN. Leprosy type 1 reactions and erythema nodosum leprosum. An Bras Dermatol 2008;83:75-82.
[Google Scholar]
|
| 9. |
Walker Sl, Lockwood DN. Leprosy Type 1 (reversal) reactions and their management. Lepr Rev 2008;79:372-86.
[Google Scholar]
|
| 10. |
Verhagen CE, Wierenga EA, Buffing AA, Chand MA, Faber WR, Das PK. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: A follow-up study. J Immunol 1997;159:4474-83.
[Google Scholar]
|
| 11. |
Van Brakel WH, Nicholls PG, Wilder-Smith EP, Das L, Barkataki P, Lockwood DN. Early Diagnosis of neuropathy in leprosy-comparing diagnostic tests in a large prospective study (the INFIR cohort study). PLoS Negl Trop Dis 2008;2:e212.
[Google Scholar]
|
| 12. |
Lockwood DN, Lukas SB, Desikan KV, Ebenezer G, Suneetha S, Nicholls P. The histological diagnosis of leprosy type 1 reactions: Identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol 2008;61:595-600.
[Google Scholar]
|
| 13. |
Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type 1 (reversal) reactions. Am J Trop Med Hyg 2002;66:409-15.
[Google Scholar]
|
| 14. |
Sousa AL, Stefani MM, Pereira GA, Costa MB, Rebello PF, Gomes MK, et al. Mycobacterium leprae DNA associated with Type 1 reactions in single lesion paucibacillary leprosy treated with single dose rifampin, ofloxacin, and minocycline. Am J Trop Med Hyg 2007;77:829-33.
[Google Scholar]
|
| 15. |
Sharma R, Lavania M, Chauhan DS, Katoch K, Amresh, Pramod, et al. Potential of a metabolic gene (accA3) of M. leprae as a marker for leprosy reactions. Indian J Lepr 2009;81:141-8.
[Google Scholar]
|
| 16. |
Lini N, Shankernarayan NP, Dharmalingam K. Quantitative real-time PCR analysis of Mycobacterium leprae DNA and mRNA in human biopsy material from leprosy and reactional cases. J Med Microbiol 2009;58:753-9.
[Google Scholar]
|
| 17. |
Zaheer SA, Beena KR, Kar HK, Sharma AK, Misra RS, Mukherjee A, et al. Addition of immunotherapy with Mycobacterium w vaccine to multi-drug therapy benefits multibacillary leprosy patients. Vaccine 1995;13:1102-10.
[Google Scholar]
|
| 18. |
Wilkinson RJ, Lockwood DN. Antigenic trigger for type 1 reaction in leprosy. J Infect 2005;50:242-3.
[Google Scholar]
|
| 19. |
Andersson AK, Chaduvula M, Atkinson SE, Khanolkar-Young S, Jain S, Suneetha L, et al. Effects of prednisolone treatment on cytokine expression in patients with leprosy type 1 reactions. Infect Immun 2005;73:3725-33.
[Google Scholar]
|
| 20. |
Kumar B, Dogra S, Kaur I. Epidemiological characteristics of leprosy reactions: 15 years experience from north India. Int J Lepr Other Mycobact Dis 2004;72:125-33.
[Google Scholar]
|
| 21. |
Mastrangelo G, da Silva Neto J, da Silva GV, Scoizzato L, Fadda E, Dallapicola M, et al. Leprosy reactions: The effect of gender and household contacts. Mem Inst Oswaldo Cruz 2011;106:92-6.
[Google Scholar]
|
| 22. |
Schreuder PA. The occurrence of reactions and impairments in leprosy: Experience in the leprosy control program of three provinces in northeastern Thailand, 1987-1995 [correction of 1978-1995]. II. Reactions. Int J Lepr Other Mycobact Dis 1998;66:159-69.
[Google Scholar]
|
| 23. |
Motta AC, Furini RB, Simão JC, Vieira MB, Ferreira MA, Komesu MC, et al. Could leprosy reaction episodes be exacerbated by oral infections?. Rev Soc Bras Med Trop 2011;44:633-5.
[Google Scholar]
|
| 24. |
Kahawita IP, Walker SL, Lockwood DN. Leprosy type 1 reactions and erythema nodosum leprosum. An Bras Dermatol 2008;83:75-82.
[Google Scholar]
|
| 25. |
Godal T, Lofgren M, Negassi K. Immune response to M. leprae of healthy leprosy contacts. Int J Lepr Other Mycobact Dis 1972;40:243-50.
[Google Scholar]
|
| 26. |
Texereau J, Chiche JD, Taylor W, Choukroun G, Comba B, Mira JP. The importance of Toll-like receptor 2 polymorphisms in severe infections. Clin Infect Dis 2005;41:S408-15.
[Google Scholar]
|
| 27. |
Bochud PY, Hawn TR, Siddiqui MR, Saunderson P, Britton S, Abraham I, et al. Toll-Like Receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J Infect Dis 2008;197:253-61.
[Google Scholar]
|
| 28. |
Bochud PY, Sinsimer D, Aderem A, Siddiqui MR, Saunderson P, Britton S, et al. Polymorphisms in toll-like receptor 4 (TLR4) are associated with protection against leprosy. Eur J Clin Microbiol Infect Dis 2009;28:1055-65.
[Google Scholar]
|
| 29. |
Walker SL, Roberts CH, Atkinson SE, Khadge S, Macdonald M, Neupane KD, et al. The effect of systemic corticosteroid therapy on the expression of toll-like receptor 2 and toll-like receptor 4 in the cutaneous lesions of leprosy Type 1 reaction. Br J Dermatol 2012;167:29-35.
[Google Scholar]
|
| 30. |
Misch EA, Macdonald M, Ranjit C, Sapkota BR, Wells RD, Siddiqui MR, et al. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl Trop Dis 2008;2:e231.
[Google Scholar]
|
| 31. |
Schuring RP, Hamann L, Faber WR, Pahan D, Richardus JH, Schumann RR, et al. Polymorphism N248S in the human Toll-like receptor 1 gene is related to leprosy and leprosy reactions. J Infect Dis 2009;199:1816-9.
[Google Scholar]
|
| 32. |
Geluk A, Ottenhoff TH. HLA and leprosy in the pre and postgenomic eras. Hum Immunol 2006;67:439-45.
[Google Scholar]
|
| 33. |
Sousa AL, Fava VM, Sampaio LH, Martelli CM, Costa MB, Mira MT, et al. Genetic and immunological evidence implicates interleukin 6 as a susceptibility gene for leprosy type 2 reaction. J Infect Dis 2012;205:1417-24.
[Google Scholar]
|
| 34. |
Moubasher AD, Kamel NA, Zedan H, Raheem DD. Cytokines in leprosy, I. Serum cytokine profile in leprosy. Int J Dermatol 1998;37:733-40.
[Google Scholar]
|
| 35. |
Britton WJ, Lockwood DN. Leprosy. Lancet 2004;363:1209-19.
[Google Scholar]
|
| 36. |
Cooper CL, Mueller C, Sinchaisri TA, Pirmez C, Chan J, Kaplan G, et al. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med 1989;169:1565-81.
[Google Scholar]
|
| 37. |
Yamamura M, Wang XH, Ohmen JD, Uyemura K, Rea TH, Bloom BR, et al. Cytokine patterns of immunologically mediated tissue damage. J Immunol 1992;149:1470-5.
[Google Scholar]
|
| 38. |
Sreenivasan P, Misra RS, Wilfred D, Nath I. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology 1998;95:529-36.
[Google Scholar]
|
| 39. |
Flesch IE, Hess JH, Kaufmann SH. NADPH diaphorase staining suggests a transient and localized contribution of nitric oxide to host defence against an intracellular pathogen in situ. Int Immunol 1994;6:1751-7.
[Google Scholar]
|
| 40. |
Lee DJ, Li H, Ochoa MT, Tanaka M, Carbone RJ, Damoiseaux R, et al. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J Infect Dis 2010;201:558-69.
[Google Scholar]
|
| 41. |
Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 2007;148:32-46.
[Google Scholar]
|
| 42. |
Martiniuk F, Giovinazzo J, Tan AU, Shahidullah R, Haslett P, Kaplan G, et al. Lessons of leprosy: The emergence of TH17 cytokines during type II reactions (ENL) is teaching us about T-cell plasticity. J Drugs Dermatol 2012;11:626-30.
[Google Scholar]
|
| 43. |
Means TK, Jones BW, Schromm AB, Shurtleff BA, Smith JA, Keane J, et al. Differential effects of a toll-like receptor antagonist on Mycobacterium tuberculosis- induced macrophage responses. J Immunol 2001;166:4074-82.
[Google Scholar]
|
| 44. |
Hernandez Mde O, Fulco Tde O, Pinheiro RO, Pereira Rde M, Redner P, Sarno EN, et al. Thalidomide modulates Mycobacterium leprae-induced NF- κ B pathway and lower cytokine response. Eur J Pharmacol 2011;670:272-9.
[Google Scholar]
|
| 45. |
Madan NK, Agarwal K, Chander R. Serum cytokine profile in leprosy and its correlation with clinic-histopathological profile. Lepr Rev 2011;82:371-82.
[Google Scholar]
|
| 46. |
Teles RM, Moraes MO, Geraldo NT, Salles AM, Sarno EN, Sampaio EP. Differential TNF alpha mRNA regulation detected in the epidermis of leprosy patients. Arch Dermatol Res 2002;294:355-62.
[Google Scholar]
|
| 47. |
Saunders BM, Cooper AM. Restraining mycobacteria: Role of granulomas in mycobacterial infections. Immunol Cell Biol 2000;78:334-41.
[Google Scholar]
|
| 48. |
Little D, Khanolkar-Young S, Coulthart A, Suneetha S, Lockwood DN. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect Immunol 2001;69:3413-7.
[Google Scholar]
|
| 49. |
Lockwood DN, Suneetha L, Sagili KD, Chaduvula MV, Mohammed I, van Brakel W, et al. Cytokine and protein markers of leprosy reactions in skin and nerves: Baseline results for the North Indian INFIR Cohort. PLoS Negl Trop Dis 2011;5:e1327.
[Google Scholar]
|
| 50. |
Fiallo P, Clapasson A, Favre A, Pesce C. Overexpression of vascular expression of vascular endothelial growth factor and its endothelial cell receptor KDR in type 1 leprosy reaction. Am J Trop Med Hyg 2002;66:180-5.
[Google Scholar]
|
| 51. |
Pesce C, Grattarola M, Menini S, Fiallo P. Cyclooxygenase 2 expression in vessels and nerves in reversal reaction leprosy. Am J Trop Med Hyg 2006;74:1076-7.
[Google Scholar]
|
| 52. |
Kirkaldy AA, Musonda AC, Khanolkhar-Young S, Suneetha S, Lockwood DN. Expression of CC and CXC chemokines and chemokine receptors in human leprosy skin lesions. Clin Exp Immunol 2003;134:447-53.
[Google Scholar]
|
| 53. |
Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol 2001;78:57-110.
[Google Scholar]
|
| 54. |
Scollard DM, Chaduvula MV, Martinez A, Fowlkes N, Nath I, Stryjewska BM, et al. Increased CXC ligand 10 levels and gene expression in type 1 leprosy reactions. Clin Vaccine Immunol 2011;18:947-53.
[Google Scholar]
|
| 55. |
Teles RM, Teles RB, Amadeu TP, Moura DF, Mendonça-Lima L, Ferreira H, et al. High matrix metalloproteinase production correlates with immune activation and leukocyte migration in leprosy reactional lesions. Infect Immun 2010;78:1012-21.
[Google Scholar]
|
| 56. |
Stefani MM, Guerra JG, Sousa AL, Costa MB, Oliveira ML, Martelli CT, et al. Potential plasma markers of type 1 and type 2 leprosy reactions: A preliminary report. BMC Infect Dis 2009;9:75.
[Google Scholar]
|
| 57. |
Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin Exp Immunol 1991;84:103-8.
[Google Scholar]
|
| 58. |
Tung KS, Umland E, Matzner P, Nelson K, Schauf V, Rubin L. Soluble serum interleukin 2 receptor levels in leprosy patients. Clin Exp Immunol 1987;69:10-5.
[Google Scholar]
|
| 59. |
Jadhav R, Suneetha L, Kamble R, Shinde V, Devi K, Chaduvula MV, et al. Analysis of antibody and cytokine markers for leprosy nerve damage and reactions in the INFIR cohort in India. PLoS Negl Trop Dis 2011;5:e977.
[Google Scholar]
|
| 60. |
Chaitanya S, Lavania M, Turankar RP, Karri SR, Sengupta U. Increased serum circulatory levels of interleukin 17f in type 1 reactions of leprosy. J Clin Immunol 2012;32:1415-20.
[Google Scholar]
|
| 61. |
Milne CD, Paige CJ. IL-7: A key regulator of B lymphopoiesis. Semin Immunol 2006;18:20-30.
[Google Scholar]
|
| 62. |
Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev 2006;211:154-63.
[Google Scholar]
|
| 63. |
Gupta N, Shankernarayan NP, Dharmalingam K. Alpha1-acid glycoprotein as a putative biomarker for monitoring the development of the type II reactional stage of leprosy. J Med Microbiol 2010;59:400-7.
[Google Scholar]
|
| 64. |
Skoff AM, Lisak RP, Bealmear B, Benjamins JA. TNF-alpha and TGF-beta act synergistically to kill Schwann cells. J Neurosci Res 1998;53:747-56.
[Google Scholar]
|
| 65. |
Spierings E, De Boer T, Zulianello L, Ottenhoff TH. Novel mechanisms in the immunopathogenesis of leprosy nerve damage: The role of Schwann cells, T cells and Mycobacterium leprae. Immunol Cell Biol 2000;78:349-55.
[Google Scholar]
|
| 66. |
Oliveira RB, Ochoa MT, Sieling PA, Rea TH, Rambukkana A, Sarno EN, et al. Expression of toll-like receptor 2 on human schwann cells: A mechanism of nerve damage in leprosy. Infect Immun 2003;71:1427-33.
[Google Scholar]
|
| 67. |
Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 2002;296:927-31.
[Google Scholar]
|
| 68. |
Smith WC, Nicholls PG, Das L, Barkataki P, Suneetha S, Suneetha L, et al. Predicting neuropathy and reactions in leprosy at diagnosis and before incident events-results from the INFIR cohort study. PLoS Negl Trop Dis 2009;3:e500.
[Google Scholar]
|
| 69. |
Ng V, Zanazzi G, Timpl R, Talts JF, Salzer JL, Brennan PJ, et al. Role of the cell wall phenolic-glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell 2000;103:511-24.
[Google Scholar]
|
| 70. |
Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health 1999;4:493-8.
[Google Scholar]
|
| 71. |
Andersson AK, Atkinson SE, Khanolkar-Young S, Chaduvula M, Jain S, Suneetha L, et al. Alteration of the cortisol-cortisone shuttle in leprosy type 1 reactions in leprosy patients in Hyderabad, India. Immunol Lett 2007;109:72-5.
[Google Scholar]
|
| 72. |
Hernandez MO, Neves I, Sales JS, Carvalho DS, Sarno EN, Sampaio EP. Induction of apoptosis in monocytes by Mycobacterium leprae in vitro: A possible role for tumour necrosis factor-alpha. Immunology 2003;109:156-64.
[Google Scholar]
|
| 73. |
Ajith C, Gupta S, Radotra BD, Arora SK, Kumar B, Dogra S, et al. Study of apoptosis in skin lesions of leprosy in relation to treatment and lepra reactions. Int J Lepr Other Mycobact Dis 2005;73:269-76.
[Google Scholar]
|
| 74. |
Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol 1997;16:83-92.
[Google Scholar]
|
| 75. |
Giacoia-Gripp CB, Sales AM, Nery JA, Santos-Oliveira JR, de Oliveira AL, Sarno EN et al. Evaluation of cellular phenotypes implicated in immunopathogenesis and monitoring immune reconstitution inflammatory syndrome in HIV/Leprosy cases. PLoS One 2011;6:e28735.
[Google Scholar]
|
| 76. |
Resino S, Bellón JM, Gurbindo MD, Muñoz-Fernández MA. CD38 expression in CD8+T cells predicts virological failure in HIV type 1-infected children receiving antiretroviral therapy. Clin Infect Dis 2004;38:412-7.
[Google Scholar]
|
| 77. |
World Health Organization. Global strategy for further reducing leprosy burden and sustaining leprosy control activities (2006-2010): Operational guidelines. Available from: http://www.who.int/lep/resources/SEAGLP20062.pdf [Last accessed on 2007 Dec 12].
[Google Scholar]
|
| 78. |
Van Veen NH, Nicholls PG, Smith WC, Richardus JH. Corticosteroids for treating nerve damage in leprosy. A Cochrane review. Lepr Rev 2008;79:361-71.
[Google Scholar]
|
| 79. |
Rao PS, Sugamaran DS, Richard J, Smith WC. Multi-centre, double blind, randomized trial of three steroid regimens in the treatment of type-1 reactions in leprosy. Lepr Rev 2006;77:25-33.
[Google Scholar]
|
| 80. |
Marlowe SN, Hawksworth RA, Butlin CR, Nicholls PG, Lockwood DN. Clinical outcomes in a randomized controlled study comparing azathioprine and prednisolone versus prednisolone alone in the treatment of severe leprosy type 1 reactions in Nepal. Trans R Soc Trop Med Hyg 2004;98:602-9.
[Google Scholar]
|
| 81. |
Sena CB, Salgado CG, Tavares CM, Da Cruz CA, Xavier MB, Do Nascimento JL. Cyclosporine A treatment of leprosy patients with chronic neuritis is associated with pain control and reduction in antibodies against nerve growth factor. Lepr Rev 2006;77:121-9.
[Google Scholar]
|
| 82. |
Lockwood DN. The management of erythema nodosum leprosum: Current and future options. Lepr Rev 1996;67:253-9.
[Google Scholar]
|
| 83. |
Tadesse A, Abebe M, Bizuneh E, Mulugeta W, Aseffa A, Shannon EJ. Effect of thalidomide on the expression of TNF-alpha m-RNA and synthesis of TNF-alpha in cells from leprosy patients with reversal reaction. Immunopharmacol Immunotoxicol 2006;28:431-41.
[Google Scholar]
|
| 84. |
Verma KK, Srivastava P, Minz A, Verma K. Role of azathioprine in preventing recurrences in a patient of recurrent erythema nodosum leprosum. Lepr Rev 2006;77:225-9.
[Google Scholar]
|
| 85. |
Kar BR, Babu R. Methotrexate in resistant ENL. Int J Lepr Other Mycobact Dis 2004;72:480-2.
[Google Scholar]
|
| 86. |
Mahajan PM, Jadhav VH, Patki AH, Jogaikar DG, Mehta JM. Oral zinc therapy in recurrent erythema nodosum leprosum: A clinical study. Indian J Lepr 1994;66:51-7.
[Google Scholar]
|
| 87. |
Faber WR, Jensema AJ, Goldschmidt WF. Treatment of recurrent erythema nodosum leprosum with infliximab. N Engl J Med 2006;355:739.
[Google Scholar]
|
| 88. |
Andersson AK, Chaduvula M, Atkinson SE, Khanolkar-Young S, Jain S, Suneetha L, et al. Effects of prednisolone treatment on cytokine expression in patients with leprosy type 1 reactions. Infect Immun 2005;73:3725-33.
[Google Scholar]
|
Fulltext Views
14,882
PDF downloads
3,693





