Translate this page into:
New insights into leukotrichia in nonsegmental vitiligo: A cross-sectional study
Correspondence Address:
Maha Fathy Elmasry
Department of Dermatology, Faculty of Medicine, Cairo University, Cairo
Egypt
| How to cite this article: Mogawer RM, Elmasry MF, Mostafa WZ. New insights into leukotrichia in nonsegmental vitiligo: A cross-sectional study. Indian J Dermatol Venereol Leprol 2019;85:374-379 |
Abstract
Background: Leukotrichia has been considered a predictor of poor outcome in vitiligo. However, studies considering the different clinical aspects of leukotrichia in vitiligo patients are few.
Aim: Our aim was to conduct a detailed clinical study to provide insights into the relevance and associations of leukotrichia in non-segmental vitiligo.
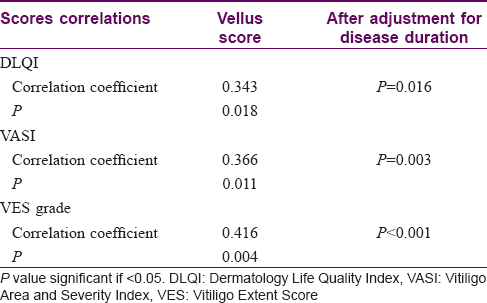
Methods: In this cross-sectional study, vitiligo patients attending the dermatology outpatient clinic and phototherapy unit at Cairo University Hospital over a period of 6 months (April–September 2016) were included. Family history, clinical details, the Vitiligo Global Issues Consensus Conference classification, the Dermatology Life Quality Index, Vitiligo Area and Severity Index, Vitiligo Extent Score, Vitiligo Disease Activity Score and Vellus Score were determined and these measurements were correlated to leukotrichia.
Results: Out of the 101 patients studied, leukotrichia was found in 47 (46.5%) patients, with vellus hair involved in 37 (78.7%), terminal hairs in 30 (63.8%) and both in 20 (42.5%) patients. Vellus hair involvement was significantly higher in generalized bilaterally symmetrical vitiligo than in acrofacial or unclassified vitiligo. The incidence of scalp leukotrichia also was higher in generalized symmetrical vitiligo than in acrofacial vitiligo. The Vellus Score showed significant associations with Vitiligo Area and Severity Index, Vitiligo Extent Score and the Dermatology Life Quality Index.
Limitations: This was a short-term study with a small sample size. Prognostic and therapeutic correlations were not studied; prospective longitudinal studies are needed for further evaluation.
Conclusion: Leukotrichia was found in almost half of the studied sample and its frequency varied among the different types of vitiligo.
Introduction
Humans have long been preoccupied with hair color and it can have a considerable psychological impact.[1] 'Leukotrichia' means 'white hair',[2] while poliosis is the presence of white or hypopigmented hairs in a group of follicles. Leukotrichia/poliosis results from a reduction or absence of melanin in the hair follicle.[3],[4] The term 'canities' is usually used to indicate progressive loss of hair pigment with age.[5]
Leukotrichia/poliosis is known to occur in a number of genodermatoses as well as diverse acquired disorders.[4],[6] In addition, normal ageing is associated with leukotrichia.[5] Leukotrichia can be classified according to the underlying etiology into genetic and acquired.[4],[6] Alternatively, leukotrichia can be classified based on its extent into localized as in vitiligo, alopecia areata and piebaldism, or generalized as in oculocutaneous albinism.[4]
Vitiligo is a well-known cause of leukotrichia where depigmentation involves the skin as well as body hair, whether vellus or terminal.[7] Leukotrichia may have major psychological effects in vitiligo patients, and is assumed to be of a similar concern as vitiliginous macules, especially in Asians.[2],[8] However, literature on leukotrichia in vitiligo is scanty.
The incidence of leukotrichia has been reported to range from 9 to 48.4% in vitiligo patients,[9] and it is observed in a high percentage (49 to 100%) of segmental vitiligo patients[7],[10] It has been reported to occur even beyond the margin of vitiliginous skin in a number of patients with segmental vitiligo. Further, it can be severe enough to involve all hairs within the vitiliginous area.[11]
Vitiligo was classically known to involve epidermal melanocytes first, before attacking melanocytes of the hair bulb and resulting in leukotrichia.[12] However, follicular vitiligo is a newly described entity in which vitiligo involves the hair follicles before the skin and this process could extend beyond the vitiliginous area.[10]
The pathogenesis of leukotrichia associated with vitiligo is assumed to be the same as that of the vitiligo itself, with identical factors acting on both epidermal melanocytes and those in the hair follicle bulb. Thus, the different theories explaining vitiligo would apply to the associated leukotrichia as well,[2] including the autoimmune hypothesis whereby antibodies are believed to attack the hair bulb melanocytes.[13]
The presence of leukotrichia is generally considered an ominous sign as it indicates depletion of the melanocyte reservoir, and thus, a poorer response to treatment.[7],[14]
Methods
The current study was conducted at the outpatient clinic and phototherapy unit of the Dermatology Department, Faculty of Medicine, Cairo University (Kasr Al Ainy Teaching Hospital) over a period of 6 months It was approved by the Dermatology Research Ethical Committee. Informed consent for participation in the study and photography was obtained from each patient (ClinicalTrials.gov registration ID: NCT03402633).
Study design
This was an observational cross-sectional study.
Patients
All vitiligo patients attending the outpatient clinic at the Kasr Al Ainy Teaching Hospital as well as newly admitted patients at the phototherapy unit were assessed from April 2016 to September 2016. A total of 101 cases were recruited.
Patients included in the study were vitiligo patients with or without leukotrichia >18 years of age. Leukotrichia when seen could be within the vitiliginous areas or on the scalp, usually in bunches of a few hairs, with or without underlying vitiligo. Patients who had received any prior treatment and those with segmental vitiligo were excluded from the study.
Methodology
Detailed history was taken including age and sex; onset, duration and course (progressive, stationary or regressive) of the condition and accordingly the Vitiligo Disease Activity Score; history of associated diseases including thyroid disease, autoimmune disorders, alopecia areata, skin malignancy, photosensitivity; any previous treatment for vitiligo; and family history of vitiligo, psoriasis, eczema, thyroid disease, premature hair graying or diabetes mellitus.
Skin examination
- Skin phototype according to the Fitzpatrick chart.[15]
- Type of vitiligo according to the Vitiligo Global Issues Consensus Conference.[16]
- Extent of involvement according to hand units: a full patient hand (the palm and the volar surface of all digits) was considered as 1% of body surface area.
- Patients were assessed for evidence of koebnerization, which is the development of vitiligo at site of skin trauma[16]
- Presence or absence of leukotrichia was assessed by naked eye examination, by Wood's light to enhance leukotrichic hair, and with a hand-held dermoscope.
In patients with leukotrichia, the types and sites of affected hair were identified, the former by means of texture, hair shaft diameter, hair length and pigmentation. Vellus hair is barely palpable, very short, fine, and poorly pigmented. while terminal hair is coarse, thick, and usually pigmented.[17],[18]
Scalp leukotrichia was considered due to vitiligo and not canities in cases with:
- Leukotrichic hair within vitiliginous scalp
- Grouped leukotrichic hair with all hairs arising from a single hair follicle.
The number of affected sites with terminal hair leukotrichia was counted in each patient. In addition, for patients with vellus hair leukotrichia, we devised a Vellus Score as shown in [Table - 1].
 Table 1: Vellus Score
Table 1: Vellus Score - Vitiligo extent score[19] was calculated for each patient:
- For each patient, pictures best representing the extent of vitiligo were chosen
- For cases not fitting well with any of the pictures, there are + and − signs which respectively indicate an extent slightly more and slightly less than in the picture
- One-fourth and half of the first picture are available options
- More than 75% means more than the last picture, but not totally depigmented
- One hundred (100) % is for total depigmentation
- Finally, total body surface area involved was calculated using www.vitiligo-calculator.com.[19]
- Vitiligo Area and Severity Index was calculated for each patient.[20]
- Dermatology Life Quality Index was calculated for each patient.[21],[22]
Investigation was done by the same dermatologist in the same room. It was done in a special room with good illumination as well as feasibility for complete room blanking to examine the patient with wood's light. Entire skin was examined for each patient and data was entered in a preset proforma.
Statistical methods
Data were coded and entered using the Statistical Package for the Social Sciences (SPSS) version 23. Data was summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Kruskal-Wallis and Mann-Whitney tests. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Correlations between quantitative variables were done using Spearman correlation coefficient. Multivariate linear regression analysis was done to adjust for disease duration in relations with vellus score. Multivariate logistic regression was done to detect relation between leucotrichia and koebnerization after adjustment for disease duration. P values less than 0.05 were considered as statistically significant.
Results
Patients' data
Among the subjects studied, 49 (48.5%) patients were males and 52 (51.5%) females; their mean age was 35.69 ± 14.82 years. There was a family history of vitiligo in 22 (21.8%) patients while psychological stress of recent onset was recorded in 39 (38.6%) patients. A summary of the study patients' clinical data is shown in [Table - 2].

Leukotrichia
Leukotrichia was recorded in 47 (46.5%) patients, involving vellus hairs in 37/47 (78.7%) patients and terminal hairs in 30/47 (63.8%) patients. Both vellus and terminal hairs were involved in 20/47 (42.5%) patients. Scalp leukotrichia affected 25 (53.2%) of the 47 patients with leukotrichia. In 4/47 (8.5%) patients, the eyebrows were involved, the eyelashes in 1/47 (2.1%), the axillary and pubic hair in 2/47 (4.3%), the moustache in 4/47 (8.5%) and the beard in 4/47 (8.5%) patients.
Leukotrichia (vellus and terminal) was significantly higher in the 32/51 (62.7%) patients with generalized bilaterally symmetrical vitiligo in comparison to the 10/31 (32.3%) patients with acrofacial vitiligo and the 4/16 (25.0%) patients with unclassified vitiligo (P = 0.004). Neither of the two patients with focal vitiligo had leukotrichia. Leukotrichia was present in the single patient with universal vitiligo included in our study.
Vellus hair leukotrichia was significantly higher in the 27/51 (52.9%) patients with generalized vitiligo in comparison to the 7/31 (22.6%) patients with acrofacial vitiligo and the 2/16 (12.5%) patients with unclassified vitiligo (P = 0.002).
No significant relationship was found between terminal hair leukotrichia and the type of vitiligo. However, when different body sites (scalp, eyebrow, eyelashes, axillary and pubic hair) with terminal hair leukotrichia were considered, scalp leukotrichia was more common in generalized (19/51 (37.3%)) than in acrofacial vitiligo (3/31 (9.7%)) and the difference was statistically significant (P = 0.001).
A statistically significant relationship was found between leukotrichia and absence of koebnerization, 39/47 (52.7%) patients with leucotrichia showing absence of koebnerization (P = 0.04). After adjustment for disease duration in a logistic regression model, no significant relation between leukotrichia and koebnerization was detected. Leukotrichia was not correlated to the presence of a halo nevus, disease stability (Vitiligo Disease Activity score) or family history of vitiligo. Disease duration was significantly higher in patients with leukotrichia (145.07 ± 135.67 months) than in those without (68.25 ± 90.00 months) (P < 0.001).
Disease duration, Dermatology Life Quality Index, Vitiligo Disease Activity, Vitiligo Area and Severity Index and Vitiligo Extent Score grades were not correlated to the number of affected areas with terminal leukotrichia.
Disease duration and vitiligo disease activity were also not correlated to the Vellus Score (considering vellus hair involvement whether solely or in association with terminal leukotrichia).
However, Dermatology Life Quality Index, Vitiligo Area and Severity Index and Vitiligo Extent Score grades all had statistically significant higher values in patients with higher Vellus Score [Table - 3] and [Figure - 1], [Figure - 2]. Even after adjustment for disease duration in a multivariate linear regression model, a significant relation still existed between the Vellus Score and Dermatology Life Quality Index, Vitiligo Area and Severity Index and Vitiligo Extent Score grade.
 |
| Figure 1: Vellus Score in relation to Vitiligo Area and Severity Index |
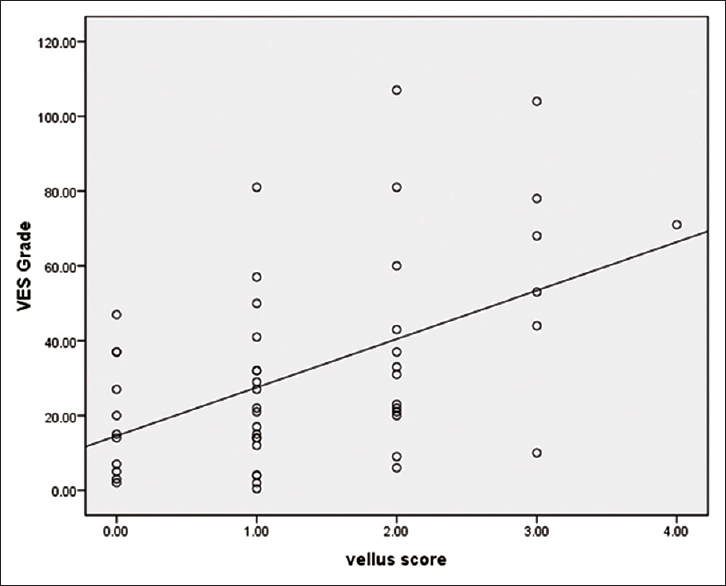 |
| Figure 2: Vellus Score in relation to Vitiligo Extent Score grade |
Dermatology Life Quality Index was significantly higher in scalp leukotrichia (mean 7.24 ± 5.2) for patients with scalp leukotrichia compared to patients without scalp leukotrichia (mean 5.32 ± 5.45) (P = 0.047), but this was not found for other areas of involvement.
We succeeded in following up only 12 of the study patients for 3 months with serial photographs. Interestingly, we noted that vellus leukotrichia repigmented in one patient in response to topical tacrolimus 0.03% applied daily for 3 months which was quite unexpected. In another patient, repigmentation of some of the affected vellus hairs occurred with improvement of associated vitiligo in response to 12 weeks (36 sessions) of treatment with narrowband ultraviolet B 311 nm. This phenomenon needs further assessment.
Discussion
Leukotrichia has always been considered a predictor of poor outcome in vitiligo patients.[7],[14],[23] One study linked the presence of leukotrichia and halo nevi at disease onset to the risk of progression from segmental vitiligo to mixed vitiligo.[16]
In 2014, Holla et al. suggested that vitiligo patients with leukotrichia are left with surgical management as the only hope.[2] However, few studies have focused on the clinical aspects of leukotrichia.
In our study group (101 patients), leukotrichia was present in 47 (46.5%) patients. We found that 32/51 (62.7%) of the patients with generalized vitiligo had leukotrichia, while 10/31 (32.3%) of those with acrofacial vitiligo and 4/16 (25.0%) with unclassified vitiligo had leukotrichia. In a study on 80 Korean children with different types of vitiligo, the incidence of leukotrichia was 25%.[24] Ihe incidence of leukotrichia reported in large groups of patients with different types of vitiligo in previous studies has varied: 1010 patients, 10%;[25] 762 patients, 33.5%%;[9] 424 patients, 9.2%,[26] and 1436 patients, 11.5%.[27] The incidence of leukotrichia in segmental vitiligo was 49% in 208 patients,[28] 100% in 82 patients,[7] and 86.1% in 188 patients[11] in different reports.
Vellus hair involvement when associated with terminal hair involvement is likely to be associated with more extensive disease and thus a higher psychological burden, as is clear from the higher Dermatology Life Quality Index values with higher Vellus Scores in our study. Moreover, we can assume that generalized bilaterally symmetrical vitiligo and vitiligo universalis, being the most extensive types of vitiligo, are more likely to develop vellus hair leukotrichia and scalp leukotrichia, a point that might aid in assessing the prognosis of these patients.
Leukotrichia was higher in patients without Koebner's phenomenon and it did not correlate with the Vitiligo Disease Activity Score in our study. Thus, leukotrichia is more likely to be observed in stable disease (Koebner's phenomenon absent) which may have followed a period of initial rapid progression. On the other hand, the Vitiligo Disease Activity Score depends on patients' perception and memory, perhaps explaining why this score did not correlate with the incidence of leukotrichia viz., 'stable disease' may not always fit into the definition of 1 year of stability. Further delineation in the context of leukotrichia is needed.
Vitiligo is well-known to be an intense psychological burden on its sufferers. We considered disease influence on the patient's quality of life in our study and found that Dermatology Life Quality Index was related to the presence of leukotrichia. Patients with higher Vellus Scores (vellus hair involvement together with terminal hair involvement) had significantly higher Dermatology Life Quality Index scores i.e. lower quality of life. This is explained by the association of more extensive disease with higher Vellus Scores, as evident from higher Vitiligo Area and Severity Index and Vitiligo Extent Score grades. On the other hand, leukotrichia in different types of hair taken globally (vellus and terminal as well as scalp leukotrichia); did not correlate with the Dermatology Life Quality Index. Thus, it may be inferred that when considering the psychological burden of the disease, it is the extent of the vitiligo itself that has a significant impact on patients' quality of life rather than the associated hair involvement. However, the Dermatology Life Quality Index was significantly higher in patients with scalp leukotrichia compared with those without scalp leukotrichia which could be explained by the fact that scalp hair is the most apparent site for leukotrichia. In addition, the Dermatology Life Quality Index was significantly higher in patients with generalized bilaterally symmetrical vitiligo in comparison to those with unclassified vitiligo. The patient's age did not have any significant influence on the Dermatology Life Quality Index. The Dermatology Life Quality Index has not been previously assessed in relation to the presence of leukotrichia or to the type of vitiligo.
Conclusion
The study gives new insights into leukotrichia in non-segmental vitiligo. We conclude that leukotrichia, whether vellus or terminal, is likely to differ among the different types of vitiligo and was higher in patients without Koebner's phenomenon. In addition, scalp leukotrichia is assumed to increase the psychological burden in vitiligo patients.
Future research is certainly needed to evaluate the phenomena of repigmentation in leukotrichic hair and melanocyte proliferation, as well as the migration markers involved. Further studies also need to look at the relationship between disease stability and leukotrichia and to confirm the hypothesis that vitiligo with leukotrichia marks aggressive disease with rapid progression and early stabilization, thus justifying the belief that surgical treatment may be the most suitable option in such patients.
Limitations of the study
This was a short-term study with only a small number of patients. Another limitation was that prognostic and therapeutic correlations were not assessed; these would need a prospective longitudinal study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Tobin DJ, Paus R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol 2001;36:29-54.
[Google Scholar]
|
| 2. |
Holla AP, Sahni K, Kumar R, Kanwar A, Mehta S, Parsad D. Repigmentation of leukotrichia due to retrograde migration of melanocytes after noncultured epidermal suspension transplantation. Dermatol Surg 2014;40:169-75.
[Google Scholar]
|
| 3. |
Roberts A, Kaye LC, Memon A, Parslew R, Kaye SB. Unilateral poliosis of the eyelashes in children associated with vitiligo. J AAPOS 2005;9:295-6.
[Google Scholar]
|
| 4. |
Sleiman R, Kurban M, Succaria F, Abbas O. Poliosis circumscripta: Overview and underlying causes. J Am Acad Dermatol 2013;69:625-33.
[Google Scholar]
|
| 5. |
Tobin DJ. The cell biology of human hair follicle pigmentation. Pigment Cell Melanoma Res 2011;24:75-88.
[Google Scholar]
|
| 6. |
Jalalat SZ, Kelsoe JR, Cohen PR. Alopecia areata with white hair regrowth: Case report and review of poliosis. Dermatol Online J 2014;20. pii: 13030/qt1xk5b26v.
[Google Scholar]
|
| 7. |
Lee DY, Kim CR, Park JH, Lee JH. The incidence of leukotrichia in segmental vitiligo: Implication of poor response to medical treatment. Int J Dermatol 2011;50:925-7.
[Google Scholar]
|
| 8. |
Al Jasser MI, Ghwish B, Al Issa A, Mulekar SV. Repigmentation of vitiligo-associated leukotrichia after autologous, non-cultured melanocyte-keratinocyte transplantation. Int J Dermatol 2013;52:1383-6.
[Google Scholar]
|
| 9. |
Agarwal S, Ojha A, Gupta S. Profile of vitiligo in Kumaun region of Uttarakhand, India. Indian J Dermatol 2014;59:209.
[Google Scholar]
|
| 10. |
Gan EY, Cario-André M, Pain C, Goussot JF, Taïeb A, Seneschal J, et al. Follicular vitiligo: A report of 8 cases. J Am Acad Dermatol 2016;74:1178-84.
[Google Scholar]
|
| 11. |
Khaitan BK, Kathuria S, Ramam M. A descriptive study to characterize segmental vitiligo. Indian J Dermatol Venereol Leprol 2012;78:715-21.
[Google Scholar]
|
| 12. |
Awad SS. Repigmentation of poliosis after epithelial grafting for vitiligo. Dermatol Surg 2013;39:406-11.
[Google Scholar]
|
| 13. |
Kim CY, Yoon TJ, Kim TH. Epidermal grafting after chemical epilation in the treatment of vitiligo. Dermatol Surg 2001;27:855-6.
[Google Scholar]
|
| 14. |
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet 2015;386:74-84.
[Google Scholar]
|
| 15. |
Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869-71.
[Google Scholar]
|
| 16. |
Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: The vitiligo global issues consensus conference. Pigment Cell Melanoma Res 2012;25:E1-13.
[Google Scholar]
|
| 17. |
Vogt A, McElwee KJ, Blume-Peytavi U. Biology of the hair follicle. In: Blume-Peytavi U, Tosti A, Whiting DA, Trueb RM, editors. Hair Growth and Disorders. Berlin: Springer; 2008. p. 1-22.
[Google Scholar]
|
| 18. |
Wang E, de Berker D, Christiano AM. Biology of hair and nails. In: Bolognia JL, Schaffer JV, Cerroni L, editors. Dermatology. 4th ed. Philadelphia: Elsevier; 2018. p. 1144-59.
[Google Scholar]
|
| 19. |
van Geel N, Lommerts J, Bekkenk M, Wolkerstorfer A, Prinsen CA, Eleftheriadou V, et al. Development and validation of the vitiligo extent score (VES): An international collaborative initiative. J Invest Dermatol 2016;136:978-84.
[Google Scholar]
|
| 20. |
Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: The Vitiligo Area Scoring Index. Arch Dermatol 2004;140:677-83.
[Google Scholar]
|
| 21. |
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – A simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6.
[Google Scholar]
|
| 22. |
Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: A comprehensive review of validation data and clinical results. Br J Dermatol 2008;159:997-1035.
[Google Scholar]
|
| 23. |
Wu XG, Xu AE. Successful treatment of vitiligo on the scalp of a 9-year-old girl using autologous cultured pure melanocyte transplantation. Pediatr Dermatol 2017;34:e22-3.
[Google Scholar]
|
| 24. |
Cho S, Kang HC, Hahm JH. Characteristics of vitiligo in Korean children. Pediatr Dermatol 2000;17:189-93.
[Google Scholar]
|
| 25. |
Vora RV, Patel BB, Chaudhary AH, Mehta MJ, Pilani AP. A clinical study of vitiligo in a rural set up of Gujarat. Indian J Community Med 2014;39:143-6.
[Google Scholar]
|
| 26. |
Shajil EM, Agrawal D, Vagadia K, Marfatia YS, Begum R. Vitiligo: Clinical profiles in Vadodara, Gujarat. Indian J Dermatol 2006;51:100-4.
[Google Scholar]
|
| 27. |
Handa S, Kaur I. Vitiligo: Clinical findings in 1436 patients. J Dermatol 1999;26:653-7.
[Google Scholar]
|
| 28. |
Hann SK, Lee HJ. Segmental vitiligo: Clinical findings in 208 patients. J Am Acad Dermatol 1996;35:671-4.
[Google Scholar]
|
Fulltext Views
16,800
PDF downloads
2,571





