Translate this page into:
Newer targeted therapies in psoriasis
Correspondence Address:
Sujay Khandpur
Department of Dermatology and Venereology, AIIMS, New Delhi - 110 029
India
| How to cite this article: Khandpur S, Bhari N. Newer targeted therapies in psoriasis. Indian J Dermatol Venereol Leprol 2013;79:47-52 |
Abstract
Psoriasis is a common, chronic, inflammatory skin disease that can have a significant impact on the quality of life of those who are afflicted due to chronicity of the disease and frequent remissions and relapses. Many available systemic therapies, however, are unsuitable for chronic administration due to the risk of cumulative toxicity. Recent advances in the understanding of the pathophysiology of psoriasis have led to the development of new, genetically engineered, targeted therapies for this disease. These include approaches targeting antigen presentation and co-stimulation, T-cell activation and leukocyte adhesion, action on pro-inflammatory mediators, and modulating the cytokine balance. Although only preliminary data are available so far and there is limited data supporting their use, these trials contribute to a further understanding of the disease and will eventually lead to new therapeutic options for psoriasis.Introduction
Psoriasis is a chronic inflammatory skin disease affecting 1-3% of the general population with psoriatic arthritis occurring in 5-40% of psoriatic cases. [1] This immune-mediated disease, which is characterized by periods of remission and relapse, produces tremendous psycho-social impact and significantly impairs the quality of life. Patients with moderate-to-severe disease require long-term therapy to control their symptoms; one-third of patients requiring systemic therapy or phototherapy. [1] Traditional systemic agents such as methotrexate, cyclosporin A, retinoids, or PUVA (Psoralen+UVA) therapy, have a potential for long-term toxicity and may not always provide sufficient improvement. Recent advances in the understanding of pathophysiology of psoriasis have led to the development of new, genetically engineered targeted therapies for psoriasis and psoriatic arthritis [Table - 1]. [2] These immune-biologics provide hope for safe and effective long-term management.
Immunopathogenesis of Psoriasis
The cells of innate immune system (keratinocytes, dendritic cells, histiocytes, neutrophils, mast cells, and endothelial cells) when activated release cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) which activate the antigen presenting cells (APCs) (Langerhans cells and resident dendritic cells) in the epidermis and dermis in genetically predisposed individuals [Figure - 1]. Trigger for this activation may be stress, infection (viral or bacterial), medication (β-blockers, lithium, chloroquine, non-steroidal anti-inflammatory drugs, tetracycline, and interferon), trauma, or an unknown trigger. The activated APCs process the antigen and migrate to regional lymph nodes where the antigen is presented to the T lymphocytes. Further differentiation of these activated lymphocytes depends on cytokines released by APCs and histiocytes, which produces IL-12 and IL-23. IL-12 favors the proliferation of Th1 and IL-23 of Th17 cells. These activated T cells then migrate to the skin through the linkage of adhesion molecules expressed in its plasma membrane-Cutaneous leukocyte antigen and Lymphocyte function -associated antigen-1 to the adhesion molecules present in the membrane of the activated cutaneous endothelial cells (E-selectin and ICAM). Th17 cells release IL17, which stimulates the keratinocytes to proliferate and produce numerous inflammatory proteins. [2] Th1 stimulation produces TNF-α and IFN-γ which are activators of transcription factors (Signal Transducer and Activator of Transcription 1 (STAT-1), STAT-3, and nuclear factor κ B) which in turn, control various groups of genes encoding inflammatory mediators in psoriasis. [3]
 |
| Figure 1: Immunopathogenesis of psoriasis |
Various targets of therapeutic action can be:
- APCs
- T cells
- Inflammatory cytokines
- Leukocyte adhesion and signal transduction
- Chemokine receptors.
Inhibitors of co-stimulation
Optimal T-cell activation requires at least two signals. One is provided by the interaction between antigen-specific T-cell receptor and antigen-major histocompatibility complexes. The other is provided by co-stimulatory signals. A well-characterized co-stimulation is mediated by CD28/B7 which is essential for T-cell proliferation following antigen stimulation; other important co-stimulatory pathways are interaction between CD2-LFA3 and ICAM-1-LFA-1 [Figure - 2]. Various experimental therapies acting on these co-stimulation pathways have shown variable success in psoriasis, which are as follows:
- LFA-3TIP (Amevive, Alefacept) blocks the interaction between LFA-3 and CD2, hampering activation of T cells, thus inhibiting further inflammatory process. [4]
- CTLA-4- Cytotoxic T- Lymphocyte Antigen 4 (CTLA-4) fusion protein (CTLA-4 Ig BMS 188667) blocks the interaction of CD80 and CD86 with CD28, further inhibiting adequate stimulation of T cells. [5]
- Anti-CD80 antibody blocks the interaction of CD80 with CD28, whereas anti-CD11a antibody blocks the interaction of LFA-1 with ICAM, thus interrupting the process of activation of T cells by APC. [4]
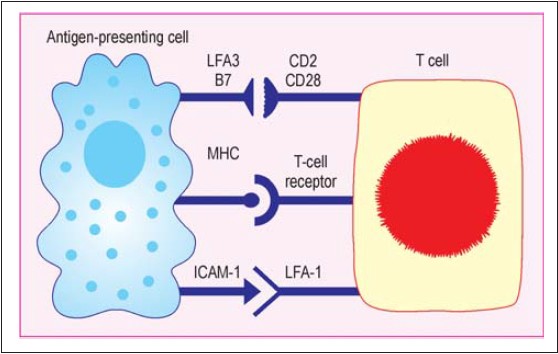 |
| Figure 2: Co-stimulation signals |
These therapies have been tried in various clinical studies with good results. With LFA-3TIP, more than half of 24 patients showed >50% improvement after 8 weeks of treatment. [4] Intravenous administration of CTLA-4 Ig led to dose-dependent improvement of >50% in 46% patients. [5] In a phase II trial of anti-CD80 monoclonal antibody in which 35 patients received four intravenous infusions of 2.5-15 mg/kg over a 3- to 36-week period, Psoriasis Area and Severity Index (PASI) 50 was achieved in 40% patients. [4]
Calcitriols
These vitamin D derivatives exert their systemic anti-psoriatic action mainly by suppressing the function of APC, in contrast to their effects after topical application where they act by direct inhibition of keratinocyte proliferation. In 85 patients, oral calcitriol (Rocaltrol) led to a decrease in PASI from 18.4 to 9.7 after 6 months and to 7.0 after 36 months. [4] But oral administration is associated with risk of hypercalcemia; hence, regular monitoring of blood and urine calcium level is essential.
T Cells
Fumaric acid esters
Methyl hydrogen fumarate, formed by hydrolysis of dimethyl fumarate (DMF), is anti-proliferative to keratinocytes. It also downregulates the production of cytokines by activated T cells and adhesion molecules on endothelial cells. Fumaderm (Biotec), an enteric-coated preparation containing DMF, calcium, magnesium, and zinc salts of monoethyl fumarate, has been used systemically for psoriasis. [6] Studies have shown that up to 50% of patients achieved PASI-75 response by 16 weeks of treatment, with many patients being maintained on this treatment for over 10 years. [7] Gastrointestinal side effects and flushing occur in up to two-thirds of patients at the induction of therapy. Lymphopenia occurs in majority of patients and impairment of renal function can rarely occur.
Anti-T-cell antibodies/fusion proteins
Monoclonal anti-CD2 (MEDI507, Siplizumab) and anti-CD3 antibodies (e.g., OKT3, HuM291), directed against all T cells, and anti-CD4 antibodies, directed predominantly against T helper cells, have been tested. [8],[9] These therapies have been reported to produce impressive and rapid improvement. Maximum effects after administration of the agent over 5-8 days were observed 2-4 weeks after the infusion.
Targeting T cells is also attempted using DAB398IL-2, an IL-2-coupled diphtheria toxin. Since IL-2 is an important growth factor for T cells, such coupling with the toxin should result in inhibition of lymphocytes. Systemic therapy with this agent in fact proved to be very effective in 40% of patients with severe psoriasis. [10] However, there were appreciable side effects, which greatly restricted its use.
Monoclonal anti-CD25 antibodies (basiliximab, daclizumab) have also been tried. Since they selectively inhibit the action of IL-2 by blocking the binding to its receptors, integrity of rest of the immune system is preserved and the patient does not suffer from consequences of immunosuppression. In an open phase II trial involving 19 patients who were given daclizumab infusions, initially 2 mg/kg and then 1 mg/kg every 2 weeks, the anti-psoriatic action was moderate, observed only in a subgroup with low psoriasis activity prior to therapy. [11] Treatment led to 30% reduction in severity after 8 weeks.
Other T-cell-specific antibodies like BT-061 (Biotest), a monoclonal CD4-specific antibody and IP10.C8 (Immune Technologies and Medicine GmbH), an inhibitor of both dipeptidylpeptidase IV and aminopeptidase N which suppress proliferation of lymphocytes and production of inflammatory cytokines of Th1, Th2, and Th17 cells are currently in phase II clinical trials. [12]
Inflammatory Cytokines
Anti-IL-8 antibodies
In a phase I-II study on 43 patients, four infusions of a humanized anti-IL-8 antibody (ABX-IL8) were given at intervals of 2-3 weeks in various doses (0-3.0 mg/kg). [4] A reduction in PASI was observed only with the higher doses, and only by 20%.
These results suggest that IL-8 does not play a key role in the pathophysiology of psoriasis and neutralizing its effects in the skin by systemic antibody therapy is difficult.
Anti-IFN
The basis of this therapy is to neutralize inflammatory type 1 cytokine, which is overexpressed in psoriasis. A humanized monoclonal antibody has recently been generated and is currently in use in a phase I-II trial. [4]
Anti-IL-12/23p40 agents
Ustekinumab (Janssen-Cilag) is a fully humanized monoclonal antibody against IL-12p40, thus inhibiting the activity of IL-12 and IL-23. [13] In a phase III trial, 121 patients with moderate-to-severe psoriasis were randomized (1:1) to receive subcutaneous injections of ustekinumab 45 mg at weeks 0, 4, 16 or placebo at weeks 0, 4 and ustekinumab 45 mg at weeks 12, 16. At week 12, the proportion of patients achieving PASI 75 was 67.2% and 5% in the ustekinumab and placebo groups, respectively (P < 0.001). [8] Ustekinumab has shown efficacy and safety in various other phase III trials also. [14]
Briakinumab (ABT-874, Abbott) is a fully human anti-IL-12/23 antibody composed of the shared p40 subunit (IL-12B) and human monoclonal IgG1 (Immunoglobulin G) heavy chain bound via a disulfide bond to a human monoclonal lambda light chain. In four phase III studies, a PASI-75 response was achieved in 80.7%, 81.9%, 80.6%, and 81.8% of briakinumab-treated patients, at week 12. [15] As a result of major cardiovascular adverse effects including myocardial infarction, stroke, and cardiovascular death, Abbott withdrew its application to the FDA (Food and Drug administration) and EMA (European Medicines Agency) for approval of the drug in July 2011. Further analyses and mechanistic studies will be required to elucidate the cardiovascular effects of briakinumab.
IL-17, IL-17 receptor, and IL-22 antagonists
Brodalumab (AMG-827, Amgen) is a fully human monoclonal anti-IL-17 receptor IgG2 antibody. A phase II dose-ranging study of brodalumab compared 70 mg, 140 mg, 210 mg, or 280 mg doses of AMG-827 twice weekly with placebo in psoriasis patients, showing a statistically significant improvement in PASI at week 12 for all dose groups compared with placebo. Two cases of neutropenia were reported in the 210 mg group. [16]
Secukinumab (AIN 475, Novartis) is a fully human monoclonal IgG1 antibody to IL-17A. A phase II trial in 36 patients treated with two doses of secukinumab, 3-10 mg/kg IV 3 weeks apart showed a 63% decrease in PASI 12 weeks after a single 3 mg/kg dose of secukinumab compared with 9% decrease in placebo-treated patients (P = 0.0005). [17]
Other monoclonal antibodies such as LY2439821 (Eli-Lilly) and RG4934 (Hoffmann-La Roche) against IL-17 and Fezakinumab (ILV-094, Pfizer) against IL-22 are in phase I trial. [18]
IL-4 administration
Increase in IL-4-leads to dominant Th2 pattern that can suppress inflammatory T-cell-mediated autoimmune processes. In an open study, 22 patients received injections of IL-4 in various doses over 6 weeks. Of 20 patients who completed the study, in 18 patients, PASI fell by 60-80% within the 6-week period. [19]
IL-10
IL-10 inhibits antigen presentation, production of inflammatory mediators, and stimulates release of anti-inflammatory mediators. In a study, IL-10, 8 ΅g/kg/day for 24 days in three cases, led to PASI reduction by 50-60% and the treatment was well tolerated. [20]
IL-11
IL-11 also possesses considerable immunosuppressant and anti-inflammatory activity. In vitro, IL-11 reduces the production of TNF-α, IL-1β, and IL-12, and in various animal models, it alleviates inflammatory activity. In a recent open study, 12 psoriasis patients were given daily subcutaneous IL-11 injections for a period of 8 weeks, of which seven showed anti-psoriatic effects, with PASI falling by 20-80%. [4]
Inhibitors of Leukocyte Adhesion
It is a humanized monoclonal anti-CD11a antibody (hu-1124, efalizumab). This antibody inhibits the interaction of CD11a (LFA-1) with various ICAM molecules. PASI 75 scores observed in Phase III studies ranged from 22% to 39% at week 12. [21] Since three patients on long-term efalizumab treatment (3 years) developed progressive multifocal leukoencephalopathy (PML), efalizumab was voluntarily withdrawn from the market in 2009.
A monoclonal anti-CD6 antibody (ior-t1) is also suggested to exert anti-psoriatic effects by inhibiting lymphocyte migration into skin, but so far there has been only one preliminary report. [22]
P-selectin glycoprotein ligand-1 (PSGL-1) is responsible for leukocyte adhesion and migration along vessel wall during inflammation and apoptosis of activated T cells late in the inflammatory response, thereby maintaining immune system homeostasis. AbGn-168 (Boehringer Ingelheim) is a humanized IgG4k antibody targeted against PSGL-1 and is in phase I trial. [23]
Signal Transduction Inhibition
Protein kinase C inhibitors
PKC family of serine/threonine kinases plays an important role in the immune signaling cascade. AEB071 (Novartis Pharmaceuticals), an oral PKC inhibitor, indirectly inhibits T-cell proliferation and prevents the production of inflammatory cytokines by activated T cells and keratinocytes. In one study, psoriatic patients receiving 300 mg twice daily of this agent showed 69% PASI improvement after just 2 weeks of treatment, with a dose-dependent inhibition of both lymphocyte proliferation and IL-2 mRNA expression. [24] Larger, long-term studies are ongoing to determine its safety and efficacy.
Mitogen-activated protein kinase inhibitors
Mitogen-activated protein kinases belong to a Ser/Thr protein kinase family and are important in cell proliferation, differentiation, inflammation, and apoptosis. A new p38 inhibitor, BMS-582949 (Bristol-Myers Squibb) has been studied in phase II trials for psoriasis. [25] This study compared the effect of 10 mg, 30 mg, or 100 mg of BMS-582949 twice daily with placebo in 99 psoriasis patients. The results are not yet available.
Phosphodiesterase 4 inhibitors
Inhibition of phosphodiesterase type 4 (PDE4) results in accumulation of cyclic adenosine monophosphate in leukocytes, and exerts anti-inflammatory effects by reducing cytokine transcription and inhibiting other inflammatory responses such as neutrophil degranulation, chemotaxis, and adhesion to endothelial cells. Apremilast (Celgene, CC-10004) is an oral PDE4 inhibitor. In a phase II clinical study of 260 psoriasis patients, a PASI-75 response was achieved with 20 mg of apremilast given twice daily in 24.4% patients after 84 days of treatment, compared to 10.3% in the placebo group. [26]
Chemokine Receptors
CXCR1 (CXCR-C-X-C chemokine receptor) and CXCR2 are G protein-coupled receptors on neutrophils, monocytes, and epithelial cells which bind to chemokines. The binding of CXCL8 and CXCL1 to CXCR1 and CXCR2, respectively, results in the release of myeloperoxidase and neutrophil chemotaxis to the epithelium. SCH527123 (Schering Plough) is an allosteric antagonist of CXCR1 and CXCR2 which inhibits neutrophil chemotaxis and release of myeloperoxidase in response to CXCL8 and CXCL1. [27]
New insights in the immunopathogenesis of psoriasis have led to discovery of numerous new therapies, though there is limited data supporting their use at the moment but many of them are under investigation and appear promising. As psoriasis is considered a life-long disease and no ultimate therapy for the disease is yet available, the need for safe and efficacious long-term treatments is of major importance. Although only preliminary data are available so far, these trials contribute to a further understanding of the disease and provide new hope for treatment of psoriasis. But risks of immunosuppression, infection, and malignancies are critical issues, so is the high cost. Also withdrawal of efalizumab due to reported cases of PML has given a lesson for cautious use of these drugs. Hence, these therapies can be used in most suited patients only. Further studies are required for more understanding of their mechanism, safety, and efficacy.
| 1. |
Kanwar AJ, Yadav S, Dogra S. Psoriasis: What is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol Venereol Leprol 2010;76:622-33.
[Google Scholar]
|
| 2. |
Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest 2004;113:1664-75.
[Google Scholar]
|
| 3. |
Sanchez AP. Immunopathogenesis of psoriasis. An Bras Dermatol 2010;85:747-9.
[Google Scholar]
|
| 4. |
Asadullah K, Volk HD, Friedrich M, Sterry W. Experimental therapies for psoriasis. Arch Immunol Ther Exp (Warsz) 2002;50:411-20.
[Google Scholar]
|
| 5. |
Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest 1999;103:1243-52.
[Google Scholar]
|
| 6. |
Ryan C, Abramson A, Patel M, Menter A. Current investigational drugs in psoriasis. Expert Opin Investig Drugs 2012;21:473-87.
[Google Scholar]
|
| 7. |
Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: A multicentre prospective randomized controlled clinical trial. Br J Dermatol 2011;164:855-61.
[Google Scholar]
|
| 8. |
Weinshenker BG, Bass BH, Ebers GC, Rice GP. Remission of psoriatic lesions with muromonab-CD3 (orthoclone OKT3) treatment. J Am Acad Dermatol 1989;20:1132-3.
[Google Scholar]
|
| 9. |
Thivolet J, Nicolas JF. Immunointervention in psoriasis with anti-CD4 antibodies. Int J Dermatol 1994;33:327-32.
[Google Scholar]
|
| 10. |
Gottlieb SL, Gilleaudeau P, Johnson R, Estes L, Woodworth TG, Gottlieb AB, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med 1995;1:442-7.
[Google Scholar]
|
| 11. |
Krueger JG, Walters IB, Miyazawa M, Gilleaudeau P, Hakimi J, Light S, et al. Successful in vivo blockade of CD25 (high-affinity interleukin 2 receptor) on T cells by administration of humanized anti-Tac antibody to patients with psoriasis. J Am Acad Dermatol 2000;43:448-58.
[Google Scholar]
|
| 12. |
Ansorge S, Bank U, Heimburg A, Helmuth M, Koch G, Tadje J, et al. Recent insights into the role of dipeptidyl aminopeptidase IV (DPIV) and aminopeptidase N (APN) families in immune functions. Clin Chem Lab Med 2009;47:253-61.
[Google Scholar]
|
| 13. |
Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol 2010;160:810-20.
[Google Scholar]
|
| 14. |
Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: A phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci 2011;63:154-63.
[Google Scholar]
|
| 15. |
Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol 2012;132:304-14.
[Google Scholar]
|
| 16. |
Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012;366:1181-9.
[Google Scholar]
|
| 17. |
Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010;2:52ra72.
[Google Scholar]
|
| 18. |
Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 2010;62:929-39.
[Google Scholar]
|
| 19. |
Thomas P. IL-4-induced immune deviation as therapy of psoriasis. Arch Dermatol Res 2001;293:39.
[Google Scholar]
|
| 20. |
Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: A new therapeutic approach. J Clin Invest 1998;101:783-94.
[Google Scholar]
|
| 21. |
Krueger J, Gottlieb A, Miller B, Dedrick R, Garovoy M, Walicke P. Anti-CD11a treatment for psoriasis concurrently increases circulating T-cells and decreases plaque T-cells, consistent with inhibition of cutaneous T-cell trafficking J Invest Dermatol 2000;115:333.
[Google Scholar]
|
| 22. |
Montero E, Falcon L, Morera Y, Delgado J, Amador JF, Perez R. CD6 molecule may be important in the pathological mechanisms of lymphocytes adhesion to human skin in psoriasis and ior t1 MAb a possible new approach to treat this disease. Autoimmunity 1999;29:155-6.
[Google Scholar]
|
| 23. |
Chen SC, Huang CC, Chien CL, Jeng CJ, Su HT, Chiang E, et al. Cross-linking of P-selectin glycoprotein ligand-1 induces death of activated T cells. Blood 2004;104:3233-42.
[Google Scholar]
|
| 24. |
Wagner J, von Matt P, Faller B, Cooke NG, Albert R, Sedrani R, et al. Structure-activity relationship and pharmacokinetic studies of sotrastaurin (AEB071), a promising novel medicine for prevention of graft rejection and treatment of psoriasis. J Med Chem 2011;54:6028-39.
[Google Scholar]
|
| 25. |
Johansen C, Vinter H, Soegaard-Madsen L, Olsen LR, Steiniche T, Iversen L, et al. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFα therapy. Br J Dermatol 2010;163:1194-204.
[Google Scholar]
|
| 26. |
Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: A randomised controlled trial. Lancet 2012;380:738-46.
[Google Scholar]
|
| 27. |
Gonsiorek W, Fan X, Hesk D, Fossetta J, Qiu H, Jakway J, et al. Pharmacological characterization of Sch527123, a potent allosteric CXCR1/CXCR2 antagonist. J Pharmacol Exp Ther 2007;322:477-85.
[Google Scholar]
|
Fulltext Views
5,176
PDF downloads
1,276





