Translate this page into:
Novel combination of oral dapsone and topical trifarotene in severe recalcitrant isotretinoin-resistant nodulocystic acne
Corresponding author: Dr. Ritu Gujarati Vishwanath, Department of Dermatology Venereology and Leprology, RiVa Skin and Plastic Surgery Clinic, Warangal, India. drrituvarun@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ritu GV, Varun V, Ramesh V. Novel combination of oral dapsone and topical trifarotene in severe recalcitrant isotretinoin-resistant nodulocystic acne. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1302_2024
Dear Editor,
Acne is a chronic inflammatory disease of the pilosebaceous unit. The pathophysiology involves three factors, namely increased sebum secretion, abnormal follicular keratinisation and Cutibacterium acnes proliferation in the pilosebaceous unit. C.acnes triggers innate immunity by causing expression of Tumour Necrosis Factor (TNF-α), peroxisome proliferator-activated receptor (PPAR), Toll-like receptors (TLR), Interferon γ (IFN), IL-8 (Interleukin), IL-12, IL-1, and matrix metalloproteinases (MMPs) by keratinocytes. An increase in IL-1 triggers keratinocyte proliferation and comedogenesis. Expression of IL-8 and Activator Protein (AP-1) further promote neutrophil chemotaxis leading to the accumulation of leukotrienes and prostaglandins.1,2 Drugs like clascoterone 1% cream, Insulin like Growth Factor 1 (IGF-1) inhibitors, PPAR modulators, topical retinoic acid metabolism-blocking agents (trifarotene), IDP-126 gel (clindamycin 1.2% + adapalene 0.15% + benzoyl peroxide 3.1% - triple combination), antimicrobial peptides and immunotherapy have evolved as novel treatment options.2-4 Laser and light-based procedures, peels are other vital therapeutic modalities for acne vulgaris. Trifarotene 0.005% is a fourth-generation synthetic retinoid for the topical treatment of acne vulgaris in patients of 9 years of age and older.4 However, isotretinoin remains the gold standard in systemic treatments. Few patients do not respond to it or have severe flares. In such cases, sarecycline, lymecycline, montelukast, spironolactone, oral contraceptive pills, TNF-α inhibitors, and oral dapsone can be used.3,5,6 We present a case of severe recalcitrant isotretinoin-resistant nodulocystic acne successfully treated with oral dapsone and topical trifarotene.
A 23-year-old man presented with severe acne on the face for one year. It was extremely painful, discharging pus and causing a great social impact. He used multiple treatments previously that gave a mild response. On examination, he had extremely tender multiple cysts, abscesses, nodules, pustules, and comedones all over the cheek, chin, forehead, and upper eyelids [Figures 1a and 1b]. The patient weighed 42 kg. The complete blood count, lipid profile, and liver and renal function tests were normal. The patient was started on isotretinoin 0.5 mg/kg, i.e., 20 mg daily at night after food. A short course of oral prednisolone 20 mg in two divided doses was given for 2 weeks along with 50 SPF sunscreen, moisturiser, and topical clindamycin. There was no response after two months. The isotretinoin dose was escalated to 30 mg per day, but the patient complained of severe cracking of lips and dryness due to which the dose was reversed to 20 mg. Intralesional triamcinolone 2 mg/ml was administered into the cysts. There was a moderate response but a rapid recurrence of new cysts. After screening for Glucose 6 Phosphate Dehydrogenase (G6PD) deficiency, he was started on dapsone (100 mg) daily at night for 1 month. Dapsone caused a appreciable response. After continuing dapsone for an additional month, the patient was prescribed topical trifarotene, moisturiser, and sunscreen (50 SPF) in the third month. Dapsone was tapered to alternate days after clearance of the lesions. The patient experienced mild dryness of cheeks, which subsided with moisturisers. The combination treatment did not cause any significant adverse effects. Dapsone was stopped tapering the dose, and trifarotene was continued. The response was very good and persistent. The Investigator’s Global Acne Assessment Score improved from 4 to 1 with minimal scarring [Figures 2a and 2b]. The patient is still in follow up.
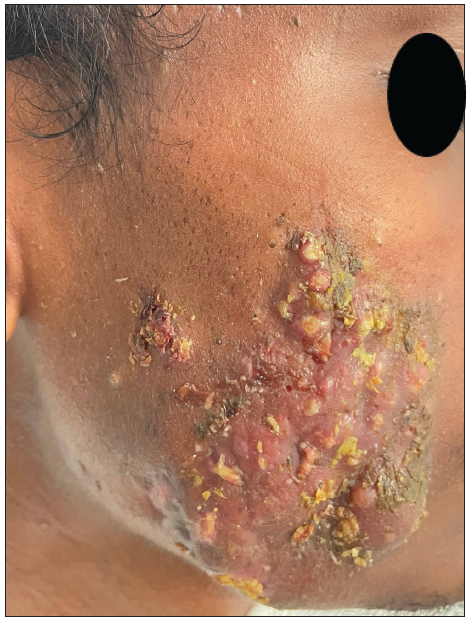
- Nodulocystic acne lesions with crusting on right cheek.
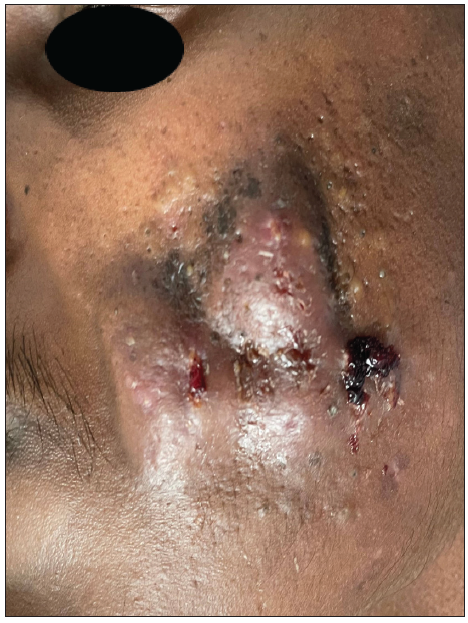
- Large nodulocystic acne lesions on left cheek.
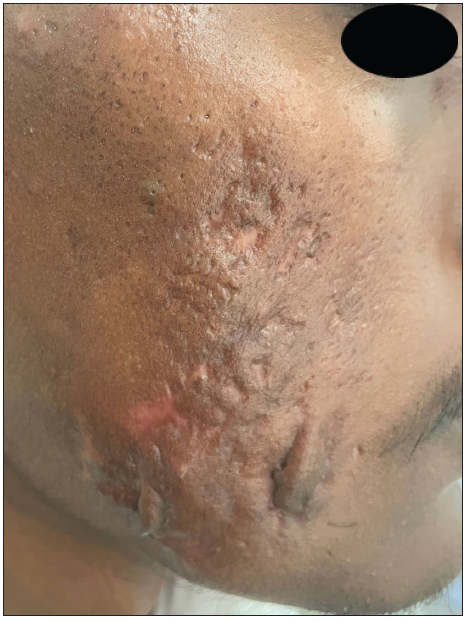
- Reduction in the Investigator’s Global Acne Assessment Score from 4 to 1 with scarring – right cheek.
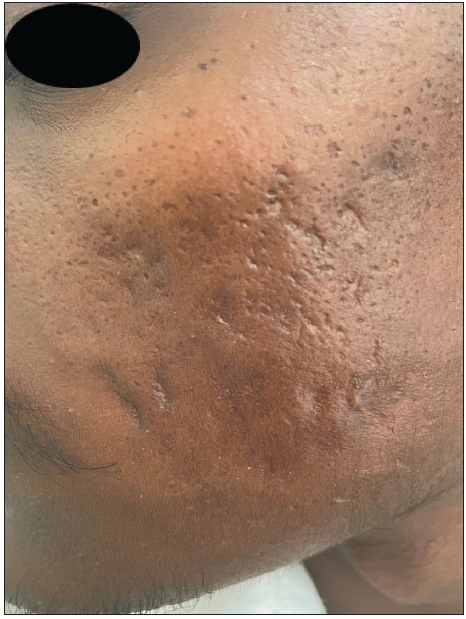
- Reduction in Investigator’s Global Acne Assessment Score from 4 to 1 with minimal scarring – left cheek.
Oral retinoids are the drugs of choice for nodulocystic acne. Most of the time, patients respond very well, but few do not respond to isotretinoin. In such recalcitrant cases, other drugs like minocycline, lymecycline, sarecycline,TNFα inhibitors, and dapsone, are useful.3,5 Dapsone’s antibacterial effect is mediated by competitive suppression of folate formation. Acne lesions are thought to be triggered by inflammation, and at the inflamed site, dapsone appears to inhibit chemo-attractant signalling, limiting neutrophil chemotaxis and lysosomal enzymes.5 Trifarotene is a novel fourth-generation retinoid that selectively agonises Retinoic Acid Receptor (RAR-γ), the predominant receptor in the epidermis. Trifarotene exerts a more targeted, skin-specific effect than earlier-generation retinoids.4 Trifarotene has a short half-life (2 to 9 hours) and does not accumulate systemically despite periodic administrations. No haematologic or biochemical abnormalities were found. Trifarotene was systemically and locally well tolerated and is safe for adults and children. Most reported adverse events like sunburn, application site irritation, and pruritus.4,6,7 Earlier, topical trifarotene was studied with doxycycline. We introduce a novel combination. No interactions were found between oral dapsone and topical trifarotene. This patient tolerated dapsone and trifarotene well. The patient stopped dapsone after 3 months and is continuing topical trifarotene till today. There is no recurrence of acne cysts. However, this is a single case report. More studies are needed to see consistent results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31:8-12.
- [Google Scholar]
- Update on etiopathogenesis and treatment of acne. Indian J Dermatol Venereol Leprol. 2017;83:298-306.
- [CrossRef] [PubMed] [Google Scholar]
- Acne vulgaris-novel treatment options and factors affecting therapy adherence: A narrative review. J Clin Med. 2022;11:7535.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trifarotene: A novel therapeutic option for acne. Dermatol Res Pract. 2022;2022:1504303.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Severe nodulocystic acne not responding to isotretinoin therapy successfully treated with oral dapsone. Oman Med J. 2018;33:433-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of acne vulgaris with trifarotene. J Cutan Med Surg. 2023;27:368-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trifarotene reduces risk for atrophic acne scars: Results from A phase 4 controlled study. Dermatol Ther (Heidelb). 2023;13:3085-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





