Translate this page into:
Onychomycosis: Clinico-mycologic study of 130 patients from Himachal Pradesh, India
2 Department of Microbiology, Indira Gandhi Medical College, Shimla, India
Correspondence Address:
Nand Lal Sharma
Department of Dermatology, Venereology and Leprosy, Indira Gandhi Medical College, Shimla - 171 001, Himachal Pradesh
India
| How to cite this article: Gupta M, Sharma NL, Kanga AK, Mahajan VK, Tegta GR. Onychomycosis: Clinico-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol 2007;73:389-392 |
Abstract
Background: Onychomycosis is a common nail infection caused by dermatophytes, yeast or other nondermatophyte molds and has diverse clinical presentations. Although common in this part of the country, no significant clinico-mycologic data is available. Objectives: This study was carried out to document the clinico-mycologic pattern of onychomycosis in Himachal Pradesh (India). Methods: All consecutive patients of onychomycosis diagnosed clinically during March 2005 to February 2006 were studied for clinical forms, number of nails involved and severity of infection. The clippings from the most severely affected nails were subjected to potassium hydroxide (KOH) mounts for direct microscopy and fungal culture on Sabouraud's dextrose agar. Results: These 130 patients (M:F 98:32) were between 8-76 years of age (mean 41.35 � 14.98 years). The prevalence of onychomycosis was higher among farmers and office workers (20% each). Finger or toe nails were exclusively involved in 56.9 and 32.3% patients respectively while these were involved concurrently in the rest of the 10.8% patients. Distal and lateral subungual onychomycosis seen in 73.1% of the specimens was the most common clinical type. KOH- and culture-positivity were recorded in 59.2 and 37.6% cases respectively. Dermatophytes and yeast (Candida albicans) were isolated in 40.8% each of the cultured nail specimens while nondermatophytic molds (NDM) were cultured in 18.6% of the samples. Various dermatophytes cultured were Trichophyton rubrum (32.6%), T. mentagrophytes (6.1%) and T. verrucosum (2.1%) respectively. Aspergillus spp. (6.1%) was the most commonly isolated NDM while other detected molds were Acremonium spp., Fusarium spp,, Scopulariopsis spp., Curvularia spp. and Penicillium marneffei. Peripheral vascular disorders (7.69%), occupational trauma (13.8%), close association with animals (60.78%) and a family history of onychomycosis (26.15%) were a few of the predisposing factors identified. Conclusion: Onychomycosis is not uncommon in this part of the country and has similar clinico-mycologic profiles in the different cases detected.




Onychomycosis comprises all fungal infections affecting the nail apparatus, i.e., nail matrix, nail plate, cuticle, mesenchymal tissue and nail folds. [1] It accounts for up to 50% of nail disorders and 30% of all superficial fungal infections of the skin. [2],[3] This may occur as a primary event or a secondary infection of a previously diseased or traumatized nail The primary pathogens are dermatophytes, nondermatophyte molds (NDM) and yeast.
Treatment of onychomycosis depends upon factors like the patient′s age and preference of regimen (daily, weekly or monthly pulse therapy), the infecting fungus and number of nails involved, degree of nail involvement, whether toenails or fingernails are affected and whether other drugs are being taken. [4] The factors contributing to recurrence may be related to the patient′s family history, occupation, lifestyle, underlying physiology or immunosuppression. [4] There are also racial and geographic variations in the prevalence of different types of fungal infections depending upon environmental, socio-economic and cultural factors. [2],[5] Thus, population-based, clinico-mycologic studies of onychomycosis appear imperative for the selection of appropriate therapy.
Himachal Pradesh, a small hill state of India in the western Himalayas, represents a distinct cohort of people who have a lifestyle, climate and environmental conditions different from those in the plains of India. Although onychomycosis is common in this part of India no significant data is available regarding this infection in this part of the country. This study was carried out to determine the clinical and etiological pattern of onychomycosis affecting the population in this part of the country.
Methods
This study was carried out over a period of one year from March 2005 to February 2006. A detailed history, clinical examination and relevant investigations of 130 consecutive patients of onychomycosis attending out Outpatient Department (OPD) were recorded. Patients who had received systemic/topical antifungal therapy in the last six months and concurrently having cutaneous or nail lesions of psoriasis, lichen planus or other dermatoses were excluded. Clinically, the disease was classified as distal and lateral subungual onychomycosis (DLSO), proximal subungual onychomycosis (PSO), white superficial onychomycosis (WSO), candidal onychomycosis (CO), endonyx onychomycosis (EO) and total dystrophic onychomycosis (TDO). The severity of nail involvement was defined on the basis of the involved area of the nail plate and the number of onychomycotic nails. Involvement was labeled as mild (≤1/3 rd of the nail plate involvement), moderate (1/3 rd -2/3 rd nail plate involved) and severe (> 2/3 rd of the nail plate involved). The most severely affected nail was thoroughly cleaned with 70% alcohol and nail clippings / scrapings were collected. A part of this sample was dissolved in 10% potassium hydroxide (KOH) and examined directly under a light microscope for fungal elements. The remainder was inoculated on (i) Sabouraud dextrose agar (SDA) supplemented with cycloheximide (100 µg/ml) and chloramphenicol (50 µg/ml) and (ii) SDA without cycloheximide supplement. These culture tubes were incubated at 37° C and 25° C respectively for 1-4 weeks. The pathogenic organisms were identified by gross colony morphology and microscopic examination of lactophenol cotton blue (LCB) mounts. Trichophyton rubrum was differentiated from other Trichophyton spp. by the urease test. [6],[7] Candida albicans was identified by Gram′s staining and the germ tube test. Nondermatophytic molds were isolated by subcultures.
Results
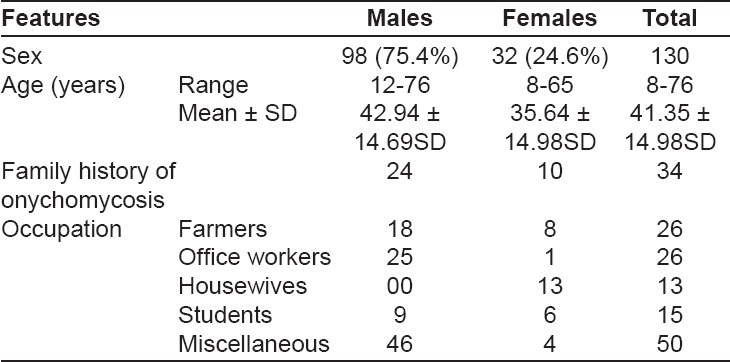
The demographic data of these 130 onychomycosis patients are shown in [Table - 1]. Thirty six (27.69%) patients aged between 31-40 years comprised the majority. [Table - 2] shows the frequency of nail involvement and clinical variants observed. Sixty eight (52.3%) patients had severe nail involvement while it was mild in 48 (36.9%) patients. The number of nails involved ranged from one to twenty. Number of nails involved were: ≤ 5 nails in 75 (57.6%) patients; 5-10 nails in 47 (36.1%) patients; 10-15 nails in 5 (4.6%) patients;> 15 nails in 2 (1.5%) patients. The only patient who had all 20 nails affected (TDO type) was HIV-positive. The fingernails on the right hand were affected in 47 (83.9%) patients while 31 (55.3%) patients had fingernail onychomycosis of the left hand. Extensive toenail involvement was seen in 82 (93%) patients of all toenail onychomycosis. The predominant nail changes observed were discoloration in 120 (92.3%), subungual hyperkeratosis in 89 (68.5%), onycholysis in 35 (26.9%), dystrophy in 49 (37.7%), leukonychia in 19 (14.6%) and paronychia in 14 (10.7%) patients respectively. Pitting, Beau′s lines and melanonychia were some of the other nail changes observed. DLSO, the most common clinical presentation seen in 95 (73.1%) patients, was followed in frequency by candidal onychomycosis in 19 (14.6%) patients. TDO and WSO with 9 (7.7%) and 6 (4.6%) patients respectively, were next in frequency. PSO was the most infrequent form; seen in only 1 (0.7%) patient who was HIV-negative. Candidal onychomycosis was seen as paronychia in 16 (84.2%) and nail plate discoloration in 17 (89.5%) of these patients. None of the patients had endonyx onychomycosis.
Ten (7.69%) patients had associated peripheral vascular disease and 18 (13.8%) patients had history of trauma. Animal handling in 79 (60.78%) and family history of onychomycosis was reported by 34 (26.15%) patients. Of the 130 specimens studied, fungal elements could be identified in 77 (59.2%) samples by KOH mounts and 49 (37.6%) specimens showed culture positivity. Both culture- and KOH-positivity were seen in 40 (30.8%) samples. Yeast and dermatophytes were isolated in 20 (40.8%) specimens each while NDM were isolated in 9 (18.4%) samples.
Various dermatophytes isolated from 14 (70%) toenail specimens include Trichophyton rubrum, T. mentagrophytes and T. verrucosum . Twelve (60%) specimens from fingernails yielded Candida albicans. Fusarium spp. , Aspergillus spp. , Curvularia spp. , Scopulariopsis spp. and Acremonium spp. were various NDM cultured from 9 (18.4%) samples (five from toenails, two from fingernails and two from both finger and toenails). One sample from fingernail onychomycosis also yielded Penicillium marneffei in culture. [Table - 3] shows various causative fungi isolated and their correlation to the clinical variants of onychomycosis.
Discussion
Onychomycosis can occur at any age but is most commonly seen during 40-60 years of age and is unusual before puberty. [5] However, peak incidence between 21-40 years of age has been observed in more recent studies. [8],[9] Incidence in both males and females has been observed, which can be attributed to the higher participation of females in outdoor activities and the use of closed footwear by men. [9],[10] The majority (83.09%) of our patients was in the 21-60 years′ age group; males being affected three times more than females. The majority (26% each of the male and female patients) were farmers and office-goers in our study. The urban patient population, as of Shimla town is more cosmetic- and health-conscious with easy accessibility to health services and generally wears closed footwear. Farmers on the other hand have increased perspiration, a greater risk of occupation-related trauma and exposure to soil saprophytes. This can explain the higher prevalence of onychomycosis in our patients involved in these occupations. Household wet work, appears to be an important predisposing factor in housewives (10%) in this report.
Toenail onychomycosis in 56.9% patients in our series was more frequent than fingernail involvement seen in 32.3% patients. This appears in consonance with previous studies particularly from countries with a temperate climate. [11] This is perhaps from wearing closed footwear for long hours due to cold weather in most months of the year as is also the case in our study. Walking long distances to their homes / work places in hilly areas further adds trauma to the nails which is a well-known predisposing factor for onychomycosis. Similarly, while working in the fields, the farmers usually wear closed rubber shoes and this combination of occlusion, perspiration, maceration and occupational trauma favors the growth of fungus. Peripheral vascular disease, occupational nail trauma, handling of animals and a family history of onychomycosis appear to be significant predisposing factors in some of our patients.
Diagnosis of onychomycosis is clinical on the basis of various changes in the affected nails. A brownish discoloration at the edge of the nail is the earliest sign when fungal infection reaches the nail plate from the nail bed. The nail bed may become thickened, cause subungual hyperkeratosis, thickening of the nail plate or onycholysis. Traumatic destruction and separation of an already diseased nail may follow. In our study, discoloration of the nails and subungual hyperkeratosis were noticed in a majority-92.3 and 68.5% of the patients respectively.
In our study, DLSO (73.1%) was the most common clinical type followed by candidal onychomycosis in 14.6% of the patients. DLSO was more commonly seen in toenails (67.36%) and candidal onychomycosis in fingernails (68.7%) conforming to clinical patterns observed in other studies. [11],[12] In candidal onychomycosis, the initial paronychia leads to nail discoloration and then, dystrophy ensues. In the present study, paronychia was seen in 84.2% of the cases and discoloration in 89.5% of the cases of candidal onychomycosis. In WSO, there is no soft keratin invasion and only nail plate leukonychia occurs. We also observed leukonychia without subungual hyperkeratosis or dystrophy in all the six patients of WSO. The severity of onychomycosis varies depending upon the size of the nail involved. In a study by Gupta et al , [11] the extent of disease was mild in 27.6%, moderate in 39.9% and severe in 32.5% of the patients. In our study, 36.9% of the patients had mild disease, 10.7% had moderate and 52.3% had severe onychomycosis. The higher prevalence of severe onychomycosis in our study can perhaps be attributed to longer duration of disease in these patients.
Direct microscopy of KOH mounts is important for clinical diagnosis while culture of the fungus is required to identify the pathogenic fungus causing the nail infection. The results of both may vary as direct microscopy is relatively easy while culture needs technical expertise. For instance, Gupta et al , [11] observed only 67.7% positivity for both the tests in their study. KOH- and culture-positivity were 59.2 and 37.6% respectively in our study, while both these tests were positive in 30.8% of the samples. Culture-positivity in the present study was 68.4% in candidal onychomycosis, 55.5% in TDO and 30.5% in DLSO.
T. rubrum has been the most common dermatophyte isolated from DLSO worldwide. [11],[12] T. rubrum also appears to be the most common dermatophyte causing DLSO in 32.6% of our patients as compared to T. mentagrophytes and T. verrucosum which were only detected in only a few patients. This high prevalence of T. rubrum has been attributed to its ability to adapt to the hard keratin of the nail. C. albicans has been reported to be the most commonly found onychomycosis isolate in many studies [8],[9] and quite frequently from cases presenting as DLSO. [11] C. albicans was the only yeast isolated in 40.8% of our samples. Apart from its isolation from 13 patients of CO, it was also isolated from four patients of DLSO, two patients of TDO and one patient of WSO in our study. Isolation of NDM in our 18.4% cases is higher than that seen in other studies. [8],[9] This could be because of frequent exposure to soil saprophytes in our patients. The most common of these isolates was the Aspergillus spp. (6.1%) seen in elderly (> 50 years) patients. Other NDM isolated were Acremonium spp. in two patients and Fusarium spp., Scopulariopsis spp , Curvularia spp. and P. marneffei in one case each. P. marneffei is a soil saprophyte causing natural infection in rodents and may also infect immunocompromised persons. [13] Our patient was a 60 year-old office worker who enjoyed gardening as a hobby. He was HIV-negative and had no other systemic illness and perhaps acquired the infection from the soil while gardening. To the best of our knowledge, he appears to be the first case of onychomycosis caused by P. marneffei in an immunocompetent individual.
As in other parts of the country, onychomycosis appears to be a common problem in Himachal Pradesh as well, with a similar clinicomycological profile. While T. rubrum and C. albicans constitute the bulk of pathogens isolated, nondermatophyte molds also were not an uncommon cause of onychomycosis in our patients.
| 1. |
Haley L, Daniel CR. Fungal infections In : Scher RK, Daniel CR, editors. Nails: Therapy Diagnosis Surgery. 1 st ed. Philadelphia: WB Saunders; 1990. p. 106-17.
[Google Scholar]
|
| 2. |
Midgley G, Moore MK. Nail infections. Dermatol Clin 1996;14:41-9.
[Google Scholar]
|
| 3. |
Richard K, Scher PK. Onychomycosis: A significant medical disorder. J Am Acad Dermatol 1996;35:S2-5.
[Google Scholar]
|
| 4. |
Gupta AK, Daniel CR. Factors that may affect response of onychomycosis to oral antifungal therapy. Aust J Dermatol 1998;59:222-4.
[Google Scholar]
|
| 5. |
Rippon JW. Dermatophytosis and dermatomycosis. In : Rippon JW, editors. Medical Mycology. 3 rd ed. Philadelphia: WB Saunders; 1988. p. 169-275.
[Google Scholar]
|
| 6. |
Baran EJ, Peterson LR, Finegold SM. Conventional and rapid microbiological methods for identification of bacteria and fungi. In : Baran EJ, Peterson LR, Finegold SM, editors. Bailey and Scotts' Diagnostic Microbiology, 9 th ed. Mosby: London; 1990. p. 106.
th ed. Mosby: London; 1990. p. 106. '>[Google Scholar]
|
| 7. |
Kane J, Fisher JB. The differentiation of Trichophyton rubrum and T. mentagrophytes by use of Christensen's urea broth. Can J Microbiol 1971;17:911-3.
[Google Scholar]
|
| 8. |
Madhuri JT, Rao GR, Lakshmi DJ. Onychomycosis: A significant medical problem. Indian J Dermatol 2002;68:326-9.
[Google Scholar]
|
| 9. |
Bokhari MA, Hussain I, Jahangir M, Haroon TS, Aman S, Khurshid K. Onychomycosis in Lahore, Pakistan. Int J Dermatol 1999;38:591-5.
[Google Scholar]
|
| 10. |
Gupta AK. Onychomycosis in the elderly. Drugs Ageing 2000;16:397-407.
[Google Scholar]
|
| 11. |
Gupta AK, Jain HC, Lynde CW, MacDonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis. J Am Acad Dermatol 2000;43:244-8.
[Google Scholar]
|
| 12. |
Ramesh V, Singh R, Reddy BSN, Kumari S. Clinico-mycological study of onychomycosis. Indian J Dermatol Venereol Leprol 1982;48:145-50.
[Google Scholar]
|
| 13. |
Hay RJ, Moore M. Mycology. In : Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 7 th ed. London: Blackwell Science Limited; 2004. p. 31.1-31.101.
th ed. London: Blackwell Science Limited; 2004. p. 31.1-31.101.'>[Google Scholar]
|
Fulltext Views
1,676
PDF downloads
1,031





