Translate this page into:
Oral ulcers and cutaneous rash as manifestations of differentiation syndrome in acute promyelocytic leukaemia
Corresponding author: Dr. Keshavamurthy Vinay, Department of Dermatology, Venereology, and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh S, Mustari AP, Lad D, Parkhi M, Vinay K. Oral ulcers and cutaneous rash as manifestations of differentiation syndrome in acute promyelocytic leukaemia. Indian J Dermatol Venereol Leprol 2023;89:449-52.
Dear Editor,
Differentiation syndrome is a well-known complication of all-trans retinoic acid (ATRA), seen in up to 27% of acute promyelocytic leukaemia (APL) patients treated with the molecule.1 It is also reported to occur with arsenic trioxide (ATO), which is co-prescribed with all-trans retinoic acid in acute promyelocytic leukaemia patients.2 It usually manifests after 5–20 days of starting treatment and the presence of three of the following criteria should rise the suspicion of differentiation syndrome; unexplained fever, weight gain (more than 5 kgs), pleuro–pericardial effusion, pulmonary infiltrates, hypotension, respiratory distress and renal failure.1 Diagnosis is primarily based on clinical presentation, after ruling out infections. Dermatological manifestations of differentiation syndrome are less known and rarely described in the literature. Herein, we report cutaneous manifestations in a case of differentiation syndrome and review the existing literature on all-trans retinoic acid-induced mucocutaneous lesions.
A 73-year-old male patient presented with progressive breathlessness, recurrent fever and ecchymoses over bilateral upper and lower limbs of 14 days’ duration. Investigation revealed pancytopenia (haemoglobin; 7.3 g/dL; total leucocyte count; 3900/mm3 and platelet count 13000/mm3). Serum lactate dehydrogenase was 3045 IU/L, prothrombin time (PT) was 17 seconds (normal 12–15), and activated partial thromboplastin time (APTT) was 32 seconds (normal 36–35). Serum electrolytes and chest radiograph were within normal limits. Bone marrow examination showed >95% promyelocytes and fluorescence in situ hybridization for t (15:17) and polymerase chain reaction (PCR) for promyelocytic leukaemia gene–retinoic acid receptor-α (PML-RARA) was positive. Diagnosis of intermediate risk acute promyelocytic leukaemia was made3 and the patient was treated with oral all-trans retinoic acid (40 mg twice daily) and intravenous arsenic trioxide (10 mg once a day) with improvement in breathlessness, fever and ecchymoses. After three weeks, patient presented with recurrence of fever and breathlessness, painful oral ulcers and swelling and redness of both upper and lower limbs. Examination revealed multiple round to oval coalescing polycyclic superficial ulcers of size 3–4 mm with yellowish base and surrounding erythematous halo over the hard palate [Figure 1]. Patient also had bilateral pitting pedal oedema extending to lower one-third of the legs with overlying diffuse erythema and scaling including soles [Figure 2]. Patient was admitted for evaluation and all-trans retinoic acid and arsenic trioxide were withheld. At admission, blood pressure was 150/88 mm Hg, pulse rate was 90 beats per minute, respiratory rate was 21 breaths per minute, temperature was 104°F and oxygen saturation was 92–97%. On auscultation, bilateral basal crepitation was present. Investigations showed anaemia (haemoglobin; 7.9 g/dL), leucocytosis (total leucocyte count; 19,900/mm3), neutrophilia (12,500/mm3), eosinophilia (1900/mm), elevated C-reactive protein (60 mg/l), D-dimer (1728 ng/mL) and creatinine (1.4 mg/dL). Arterial blood gas analysis, liver function tests, serum procalcitonin, serum galactomannan, serum fibrinogen, coagulation profile (except D-dimer) and serum electrolytes were within normal limits. Chest radiograph showed bilateral diffuse infiltrates with right pleural effusion. Computed tomography (CT) of the chest showed bilateral ground-glass opacity and mild pleural effusion. Bronchoalveolar lavage examination (gram stain, acid-fast bacteria, periodic acid–Schiff were negative) did not show evidence of an infective aetiology. The patient was started on intravenous cefoperazone-sulbactam empirically. Three blood cultures sent 24 hours apart were sterile. Tzanck smear from the oral ulcers was negative for multinucleate giant cells. Cytomegalovirus, Epstein-Barr virus, herpes simplex virus and COVID-19 PCR were negative. Skin biopsy from the leg showed hyperkeratosis and mild superficial perivascular infiltrate of lymphocytes and occasional eosinophils [Figure 3]. The patient continued to have spikes of fever; breathlessness, oral ulcers and rash persisted despite changing the antibiotics to vancomycin and colistin. all-trans retinoic acid and/or arsenic trioxide-induced differentiation syndrome was suspected based on clinical features, lack of response to antibiotics, and history of recent intake of all-trans retinoic acid and arsenic trioxide. The patient was treated with prednisolone 40 mg per day with remarkable improvement in skin and mucosal lesions. The respiratory complaints also improved and there was a significant improvement in the chest radiograph. Prednisolone tapering was started after 10 days and stopped over one month. The patient was restarted on all-trans retinoic acid and arsenic trioxide after one month without any recurrence. Naranjo adverse drug reaction probability scale score was 3 for both all-trans retinoic acid and arsenic trioxide and 6 for a combination of all-trans retinoic acid and arsenic trioxide.
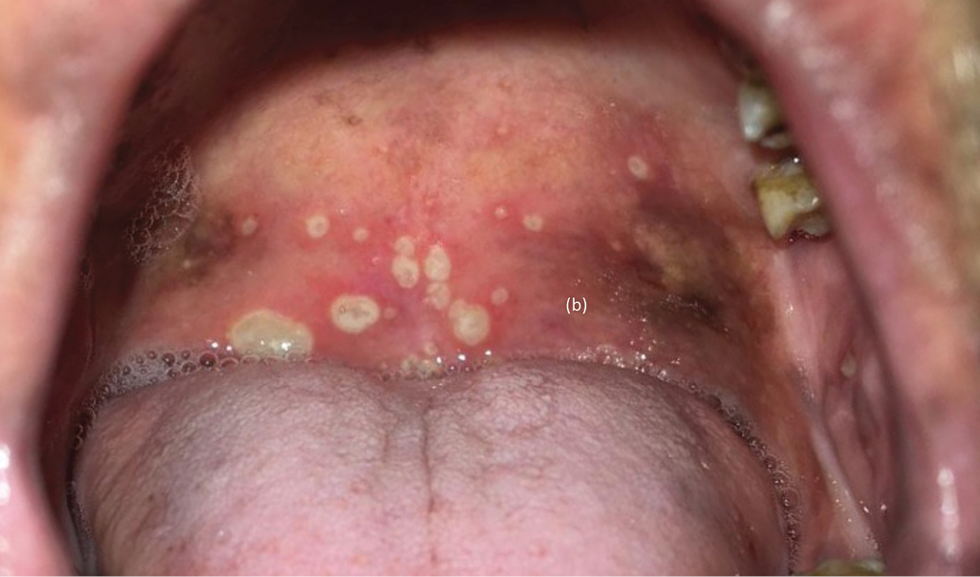
- Multiple oral ulcers with yellowish base and surrounding erythematous halo over the hard palate
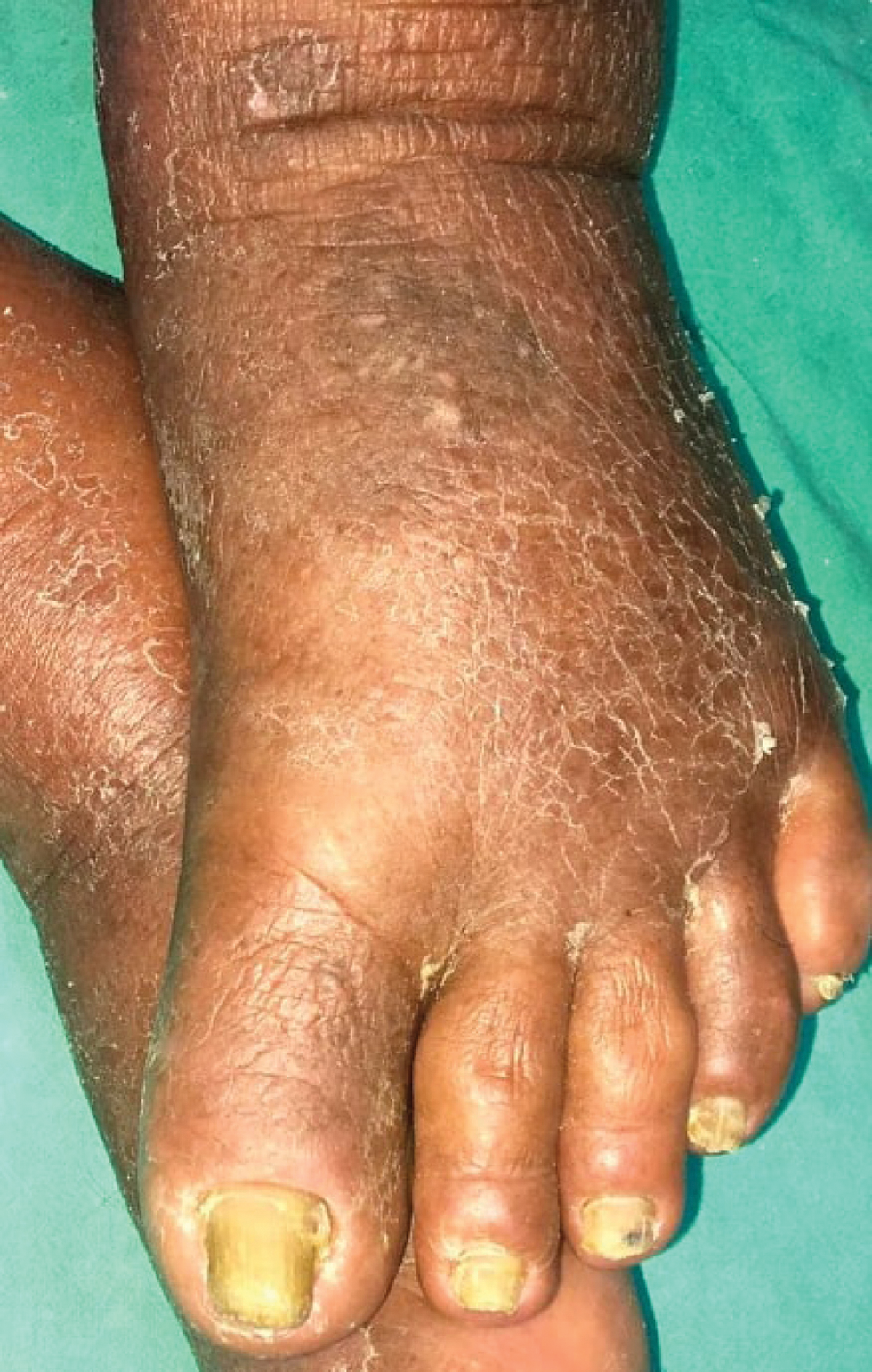
- Bilateral pedal oedema with overlying diffuse erythema and scaling
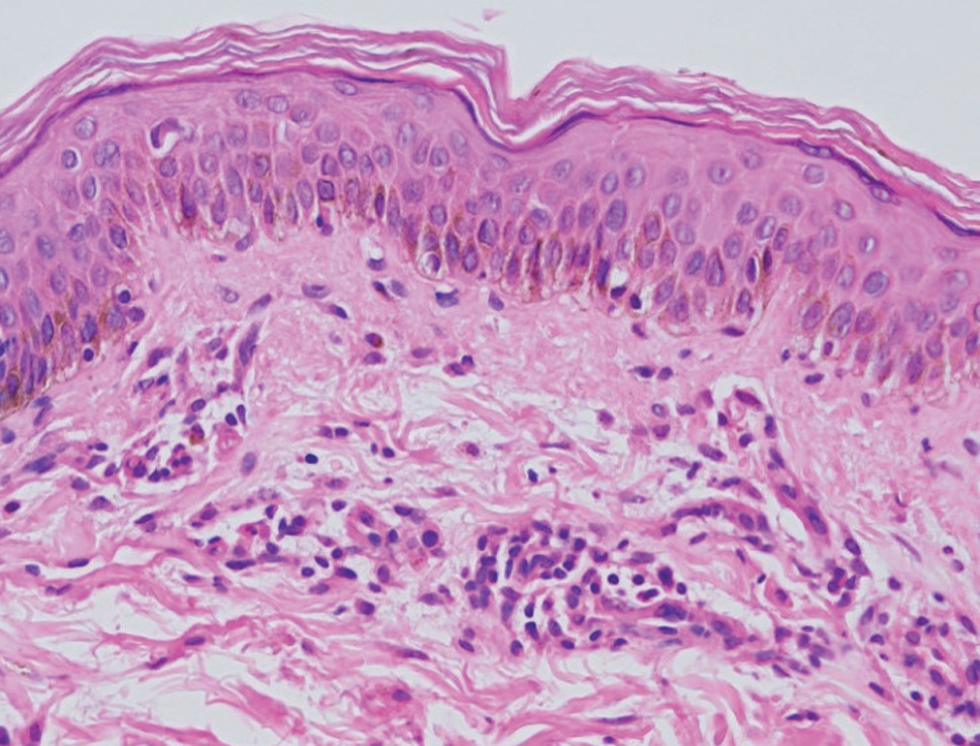
- The skin biopsy shows hyperkeratosis and superficial dermal mild perivascular infiltrate of lymphocytes and occasional eosinophils (Haematoxylin and eosin, ×;400)
Acute promyelocytic leukaemia is a type of acute myeloid leukaemia that affects both children and adults. The first line of therapy for acute promyelocytic leukaemia is a combination of all-trans retinoic acid and arsenic trioxide. all-trans retinoic acid is a vitamin A derivative used in the treatment of acute promyelocytic leukaemia which acts via a retinoic acid receptor (RAR α, β and γ) that helps in the differentiation and apoptosis of malignant cells.4 Arsenic trioxide also helps in the differentiation of malignant cells by degrading promyelocytic leukemia gene–retinoic acid receptor-α (PML-RARA).
Differentiation syndrome is a serious, life-threatening, unpredictable clinical syndrome due to the differentiation of blast cells in acute promyelocytic leukaemia patients after induction therapy with all-trans retinoic acid and/or arsenic trioxide. It has also been reported in patients of pustular psoriasis and psoriasis vulgaris treated with acitretin.5 The precise mechanism of differentiation syndrome is unknown, but the differentiating acute promyelocytic leukaemia cells are primary mediators of inflammation. These cells have an increase in adhesive property and interleukin production that helps in transmigration through vessels, infiltration into various organs and subsequent immune-mediated organ damage. This leads to capillary leak, oedema, haemorrhage, leucocyte infiltration and release of inflammatory mediators and subsequent organ failure, commonly respiratory failure.6
The risk factors for differentiation syndrome include baseline high total leucocyte count (TLC), rapidly improving TLC and CD13 expression of leukaemic cells.7 The occurrence of differentiation syndrome is associated with an increased risk of extramedullary relapse.8 Differentiation syndrome has a spectrum of cutaneous manifestations including labial and lingual ulcers, scrotal ulcers and Sweet’s syndrome. Other all-trans retinoic acid-induced cutaneous manifestations reported are exfoliative dermatitis, xerosis, xerostomia, cheilitis and ulcers over the vulva, pubis, perianal area and lower abdomen [Table 1].9-18 Riganti et al.9 reported a painful lingual ulcer as part of differentiation syndrome and there was complete resolution after three days of starting dexamethasone (8 mg/day). In contrast, our patient had multiple smaller ulcers.
| Year | Disease | Treatment received for primary disease | Clinical presentation | Cutaneous manifestation | Number of features fulfilling diagnostic criteria of DS | Treatment of cutaneous manifestations | Treatment response |
|---|---|---|---|---|---|---|---|
| 1994 | APL | ATRA, 80 mg per day | Fever and leucocytosis | Skin nodules over arms (Sweet’s syndrome) | 1 | Intravenous methylprednisolone 1 gram for three days | Improvement of skin lesions |
| 2000 | APL | ATRA, 45 mg/m2 and ADE regimen | Fever, fluid overload | Multiple scrotal ulcers | 2 | Prednisolone 40 mg | Healed after three weeks |
| 2000 | APL | ATRA 40 mg BD | Fever | Multiple scrotal ulcer | 1 | ATRA continued No mention of steroids | Ulcer healed after completion of treatment |
| 2002 | APL | ATRA, 45 mg/m2, daunorubicin 60 mg/m2/day and cytarabine 200 mg/m2/day | Fever and weight gain | Violaceous and erythematous papulo-vesicles on the limbs and upper trunk (Sweet’s syndrome) | 2 | ATRA was stopped Dexamethasone 20 mg per day | Improvement after two weeks |
| 2005 | APL | ATRA, 40 mg BD and chemotherapy | Fever, lower extremity oedema, and mild pulmonary infiltrates | Solitary scrotal ulcer | 3 | ATRA was stopped No mention of steroids | Ulcer healed shortly after stopping ATRA |
| 2011 | APL | ATRA, 45 mg/m2, daunorubicin and cytarabine | Fever, breathlessness, fatigue, pallor, tachypnoea and pancytopenia | Solitary superficial scrotal ulcer | 2 | ATRA was stopped No mention of steroids | Ulcer healed in three weeks |
| 2012 | APL | ATRA, 40 mg BD and idarubicin | Fever, weight gain, mild pleural, pericardial, and peritoneal effusion | Solitary lingual ulcer | 3 | ATRA was stopped Dexamethasone 8 mg per day | Ulcer healed after seven days |
| 2018 | APL | ATRA, 40 mg BD, cytarabine and daunorubicin | Fever and breathlessness | Painful labial ulcer with scrotal ulcer | 2 | ATRA was stopped Started on Dexamethasone (dose not mentioned) | Ulcers healed after 20 days |
| 2020 | GPP | Acitretin, 25 mg per day | Fever, facial puffiness, breathlessness and dry cough, tachycardia, dyspnoea, hypotension, oedema, bilateral basal crepitations | Generalised erythema and scaling with relative sparing of the palms and soles and periorbital oedema | 3 | ATRA was stopped Intravenous betamethasone | Improvement after 2 days |
| 2020 | APL | ATRA, 45 mg/m2 and Daunorubicin 60 mg/m2/day | Fever, breathlessness, pulmonary infiltrates and pancytopenia | Multiple coalescing scrotal ulcers | 3 | ATRA was stopped Dexamethasone 10 mg BD | Healed in four weeks |
| APL | ATRA, 40 mg BD | Fever, breathlessness, leucocytosis, pulmonary infiltrates, pleuro-pericardial effusion and elevated creatinine | Oral ulcers, pedal oedema with diffuse erythema and scaling including soles | 4 | ATRA was stopped Prednisolone 40 mg per day | Improvement after three days |
BD: twice a day, ATRA: All-trans retinoic acid, APL: acute promyelocytic leukaemia, GPP: generalised pustular psoriasis, ADE: daunorubicin, etoposide and cytarabine, DS: differentiation syndrome
Even though all-trans retinoic acid or arsenic trioxide can induce differentiation syndrome, cutaneous manifestations are exclusively reported with all-trans retinoic acid-induced differentiation syndrome. These manifestations respond well after stopping all-trans retinoic acid and starting systemic steroids. The decision to stop drugs should be tailored to individual patients and depends on the severity of differentiation syndrome. The treatment of differentiation syndrome includes dexamethasone 10 mg twice a day till resolution or for a minimum of three days.19 Prednisolone 40 mg per day has also been used to treat differentiation syndrome and same was used in index because of the lack of severe systemic symptoms.10
In our patient, the oral ulcers, pedal oedema, erythema and scaling were attributed to differentiation syndrome as these started after two weeks of starting all-trans retinoic acid and arsenic trioxide and responded dramatically to systemic steroids. Dermatologists should be aware of these cutaneous manifestations that help in the early diagnosis and better management of the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Retinoic acid syndrome: A review. J Clin Pharm Therapeut. 2008;33:331-8.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation syndrome in promyelocytic leukemia: Clinical presentation, pathogenesis and treatment. Mediterr J Hematol Infect Dis. 2011;3:e2011048.
- [CrossRef] [PubMed] [Google Scholar]
- Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133:1630-43.
- [CrossRef] [PubMed] [Google Scholar]
- All-trans retinoic acid: Tolerance and biologic effects in myelodysplastic syndrome. J Clin Oncol. 1993;11:1489-95.
- [CrossRef] [PubMed] [Google Scholar]
- Acitretin-induced retinoic acid syndrome. J Am Acad Dermatol. 2011;65:e148-9.
- [CrossRef] [PubMed] [Google Scholar]
- Retinoic acid syndrome: Manifestations, pathogenesis, and treatment. Best Pract Res Clin Haematol. 2003;16:453-61.
- [CrossRef] [PubMed] [Google Scholar]
- Role of polyamines derived from arginine in differentiation and proliferation of human blood cells. Biol Pharm Bull. 2006;29:234-9.
- [CrossRef] [PubMed] [Google Scholar]
- Extramedullary relapse after all-trans retinoic acid treatment in acute promyelocytic leukemia–the occurrence of retinoic acid syndrome is a risk factor. Leukemia. 1999;13:1406-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lingual ulceration associated with retinoic acid syndrome during treatment of acute promyelocytic leukemia. Int J Dermatol. 2014;53:912-6.
- [CrossRef] [PubMed] [Google Scholar]
- Scrotal ulceration during all-trans retinoic (ATRA) therapy for acute promyelocytic leukaemia. Clin Lab Haematol. 2000;22:171-4.
- [CrossRef] [PubMed] [Google Scholar]
- Sweet’s syndrome associated with trans-retinoic acid treatment in acute promyelocytic leukaemia. Clin Exp Dermatol. 1994;19:51-2.
- [CrossRef] [PubMed] [Google Scholar]
- All-trans-retinoic acid-induced scrotal ulcerations in a patient with acute promyelocytic leukemia. J Am Acad Dermatol. 2000;43:316-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia. Ann Hematol. 2002;81:111-4.
- [CrossRef] [PubMed] [Google Scholar]
- Scrotal ulceration induced by all-trans retinoic acid in a patient with acute promyelocytic leukemia. Int J Dermatol. 2005;44:68-9.
- [CrossRef] [PubMed] [Google Scholar]
- Scrotal ulceration following all-trans retinoic Acid therapy for acute promyelocytic leukemia. Indian J Dermatol. 2011;56:561-3.
- [CrossRef] [PubMed] [Google Scholar]
- Rare labial ulcer related to the use of all-trans retinoic acid in a patient with acute promyelocytic leukemia. Spec Care Dentist. 2018;38:234-238.
- [CrossRef] [PubMed] [Google Scholar]
- Acitretin-induced differentiation syndrome in a case of generalized pustular psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:231.
- [CrossRef] [PubMed] [Google Scholar]
- Retinoic acid syndrome followed by scrotal ulcer during treatment of acute promyelocytic leukemia with all-trans retinoic acid. J Curr Oncol. 2020;3:97-9.
- [CrossRef] [Google Scholar]
- Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021;11:123.
- [CrossRef] [PubMed] [Google Scholar]





