Translate this page into:
Ozenoxacin: A novel topical antibiotic
Corresponding author: Dr. Parvathy Santhosh, Department of Dermatology, Malabar Medical College Hospital and Research Centre, Modakallur, Kozhikode, Kerala, India. drparvathysanthosh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Santhosh P, Thomas MH. Ozenoxacin: A novel topical antibiotic. Indian J Dermatol Venereol Leprol 2021;87:131-4.
Introduction
Superficial skin infections are a common cause of visits to dermatology out-patient department, especially in the pediatric age group. Impetigo is a common infection in both children and adults, and is caused by Staphylococcus aureus and Streptococcus pyogenes.1 Localized cases of impetigo without complications may be treated with topical antibiotics, while extensive cases may need systemic therapy.2 Mupirocin and fusidic acid are the commonly used topical agents for impetigo while retapamulin is a recent alternative.3,4 Mupirocin and retapamulin are bacteriostatic at low concentrations but may be bactericidal at higher concentrations.4,5 Fusidic acid is reported to be bactericidal for S.pyogenes and bacteriostatic for S.aureus.6 Antimicrobial resistance is now becoming more prevalent, and the emergence of plasmid-mediated, mutually transferrable mupirocin resistance in Staphylococcus aureus and Staphylococcus epidermidis has also been demonstrated in vivo, reported to arise due to extensive use of mupirocin in in-patients.7,8 Although the clinical implications of mupirocin resistance are unclear, and topical mupirocin achieves high minimum inhibitory concentrations (MIC), even more than what is required to overcome high-level mupirocin resistant strains, the emergence of resistant strains necessitates the need for newer antimicrobials.9 The latest drug to join the group of topicals is ozenoxacin. In December 2017, ozenoxacin received FDA approval for use in the management of impetigo in patients aged 2 months and older.10
Structure
The molecular formula of ozenoxacin is C21H21N3O3 and the IUPAC name is 1-cyclopropyl-8-methyl-7-[5-methyl- 6-(methylamino) pyridin-3-yl]-4-oxoquinoline-3-carboxylic acid.10,11 It belongs to the quinolone group of antimicrobials and is the first non-fluorinated quinolone, which has a pyridinyl group at C7. Ozenoxacin has a structural similarity with quinoline carboxylic acids, which are quinolines where the quinoline ring system is substituted by a carboxyl group at one or more positions [Figure 1].11,12
Mechanism of Action
Quinolones act by inhibiting two enzymes involved in bacterial DNA synthesis- DNA gyrase (a type II topoisomerase) and topoisomerase IV.13 The action of DNA gyrase is to catalyze the negative supercoiling of DNA. The enzyme plays a vital role in DNA replication and transcription and in organization of the chromosome. The main function of topoisomerase IV is to unlink DNA after replication.14-16 Both enzymes have a tetrameric structure. DNA gyrase has two A subunits (gyrA, coded by the gyrA gene) and two B subunits (gyrB, coded by the gyrB gene). Topoisomerase IV also has two A subunits (parC or grlA, the latter in S. aureus, encoded by the parC or grlA genes) and two B subunits (parE or grlB, the latter in S. aureus, encoded by the parE or grlB genes).13 Some quinolones possess preferential action against topoisomerase IV over DNA gyrase (e.g. levofloxacin, sparfloxacin, nadifloxacin, and ciprofloxacin).17,18
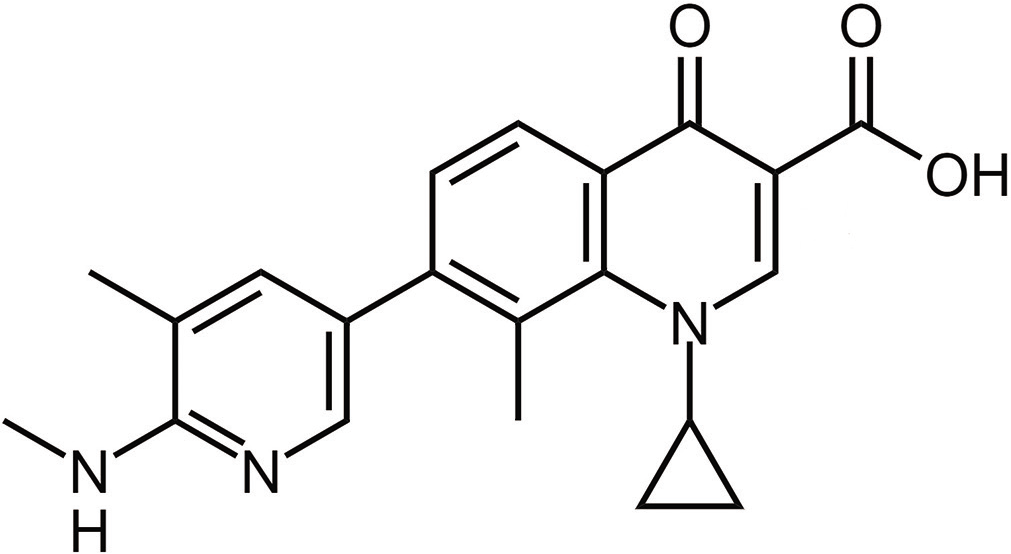
- Chemical structure of ozenoxacin
Ozenoxacin has dual inhibitory action and acts by irreversibly binding to either DNA gyrase or topoisomerase IV, thus inhibiting DNA replication. This typically results in rapid bacterial cell death and thus the drug is bactericidal.12,19
Antimicrobial Spectrum
Quinolones have broad spectrum, potent antibacterial activity and are bactericidal against both gram-positive and gram-negative organisms. Ozenoxacin has bactericidal action against gram-positive organisms including MSSA (methicillin-sensitive Staphylococcus aureus), MRSA (methicillin-resistant Staphylococcus aureus), MRSE (methicillin-resistant Staphylococcus epidermidis), Streptococcus pyogenes, Propionibacterium acnes and ofloxacin-resistant strains of S. aureus and S. epidermidis.19-21 The antibacterial activity of ozenoxacin has been found to be highly superior and the MIC lower, compared to other quinolones such as ofloxacin, nadifloxacin, and levofloxacin.20
Ozenoxacin also been demonstrated to produce more rapid microbiological clearance compared to retapamulin, although the overall clinical efficacy at the end of treatment has been reported to be similar. In animal models, ozenoxacin has demonstrated more rapid microbiological clearance compared to mupirocin and fusidic acid, although there are no comparative human trials.22-24 Ozenoxacin has also been found to have lower MIC compared to fusidic acid, mupirocin and retapamulin in vivo against S. aureus, S. epidermidis, S. pyogenes and S.agalactiae. 15,21 The drug exhibits rapid penetration inside the bacterial cell in the first minute after exposure and high levels of intrabacterial concentrations compared with other quinolones in various microorganisms.18,25 The minimum bactericidal concentrations (MBCs) have been observed to be generally the same or within one dilution of the MICs.18,21,22,26
Resistance
Acquisition of resistance to quinolones is through two mechanisms- one is through efflux pumps that pump out the drug from the bacterial cell, and the other through mutations in genes encoding DNA gyrase and topoisomerase IV.13,18,27 Resistance to quinolones is now rampant.13
Ozenoxacin appears to be impervious to the efflux pumps that confer bacterial resistance to other quinolones, which may be attributed to its high concentration inside bacterial cell. 18,25,27 It shows low selection of resistant mutants, and has a mutant prevention concentration less than the skin concentration of the drug. These mechanisms protect ozenoxacin against the development of resistance. Ozenoxacin does not show cross-resistance with other quinolones or other anti-microbial classes.18,21
Pharmacokinetics
Ozenoxacin exhibits negligible systemic absorption from both intact and abraded skin. The drug concentrations have been found to be high in the stratum corneum, low in the epidermis and below the limit of quantification in the dermis.28 There is no difference in absorption between adult and pediatric skin.22
Owing to the low systemic absorption, the tissue distribution, metabolism and elimination in humans have not been investigated in in-vivo studies.18 Mean protein binding of [14C]-ozenoxacin in in-vitro studies has been found to be moderate (~85% to 87%) in human plasma samples and independent of concentration.10 [14C]-ozenoxacin has been found to be metabolically stable in in vitro human hepatocytes and in freshly prepared human skin discs.10,26
Indications
Topical ozenoxacin is indicated for the treatment of impetigo caused by Staphylococcus aureus and Streptococcus pyogenes. 28,29 It is approved in the US and Canada for the treatment of non-bullous and bullous impetigo in adults and pediatric patients aged 2 months and older. 10,26,29,30 It has also been approved in 12 countries of the European Union (EU) for topical treatment of non-bullous impetigo in patients aged 6 months and older. 26,30,31
Composition and Storage
Ozenoxacin is available as 1% cream (10 mg/g) in 10-g tubes. It is a white to pale yellow crystalline powder, available commercially as a pale-yellow cream. It must be stored at room temperature and must be used within 45 days of opening the tube.10,26
The other ingredients used in the preparation are benzoic acid (E 210), ethylene glycol monopalmitostearate, octyldodecanol, oleoyl macrogol-6-glycerides, polyethylene glycol-6 stearate, polyethylene glycol-32 stearate, propylene glycol, purified water and stearyl alcohol. 26
Dosage and Administration
A thin layer of ozenoxacin cream must be applied to the affected area twice daily for 5 days. It can be applied over a maximum area of 100 cm2 in adults and pediatric patients 12 years of age and older. In patients less than 12 years of age, it can be applied up to 2% of the total body surface area, but not exceeding 100 cm2. The area of application may be covered by a sterile bandage or gauze dressing if required. Patients who do not show a clinical response within 3 days of treatment should be re-evaluated, and alternative therapy should be considered.26,30
Adverse Effects and Safety Profile
Ozenoxacin is considered to have an excellent safety profile and no significant adverse effects have been reported.18,28,29 There is however, a single report of worsening pre-existing rosacea and seborrheic dermatitis.29
Ozenoxacin 1% and 2% formulations have been tested for dermal tolerability and were shown to be safe with little or no tendency to produce irritation, sensitization, phototoxicity or photoallergy.32
The efficacy and safety in patients less than 2 months have not been established. No dose adjustment is necessary in geriatric patients or in hepatic or renal impairment. Studies in pregnant and breastfeeding women have not been performed, but since systemic absorption is negligible, adverse events are not expected. If ozenoxacin is used during breast-feeding, it is recommended to avoid applying on the breast area to prevent the nursing infant from unintentional oral drug intake.18,26,28
Quinolones have potential chondrotoxic effects on juvenile animals. However, no histopathological toxicologically relevant changes in cartilage were observed in rats treated with oral ozenoxacin.33 The lack of fluorination at C-6 position is considered to improve the safety profile of ozenoxacin compared to other quinolones.20 There is no systemic absorption in children, hence negating the possibility of chondrotoxicity.34
Drug Interactions
Ozenoxacin does not induce cytochrome P450 enzymes in vitro. Since systemic absorption is negligible, there are no reported drug interactions.26
Contraindications
The only known contraindications till date are patients who are hypersensitive to this drug or to any ingredient in the formulation and infants less than 2 months of age due to lack of data about safety in that age group.26,30
Cost and Cost Effectiveness
A 10-g tube of ozenoxazin costs $ 17.78 in the United states, which makes the cost $ 1.778 per gram. Fusidic acid costs $0.7340 per gram while the cost of mupirocin ranges from $0.3556 to 0.550. Although ozenoxacin was found to have a lower total cost, this was based on the assumption of a lower time-to-cure. Based on a re-analysis considering similar clinical rates of cure among topical treatments, a price reduction of 28% (vs. fusidic acid) and 51% (vs. mupirocin) would be required for ozenoxacin to be cost-effective. Many oral antibiotics are considerably less expensive than ozenoxacin. The clinical effectiveness of ozenoxacin was not compared with oral antibiotics; hence the cost-effectiveness is not known it is not yet available in India.35
Conclusion
Ozenoxacin is a newer topical antibiotic with faster clinical efficacy, favourable microbiological profile and safety. However, indiscriminate use must be avoided, and antimicrobial stewardship must be practiced to preserve the value of this novel drug.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Anti-bacterial drugs (5.1) In: British National Formulary. London: BMJ Books; 2001. p. :252-90.
- [Google Scholar]
- A systematic review and meta-analysis of treatments for impetigo. Br J Gen Pract. 2003;53:480-7.
- [Google Scholar]
- Retapamulin: A novel topical antibiotic. Indian J Dermatol Venereol Leprol. 2010;76:77-9.
- [CrossRef] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. PubChem Database Mupirocin, CID=446596. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Mupirocin [Last accessed on 2020 Jun 5]
- [Google Scholar]
- Fusidic acid: A bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb Perspect Med. 2016;6:a025437.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characterization and transfer among Staphylococcus strains of a plasmid conferring high-level resistance to mupirocin. Eur J Clin Microbiol Infect Dis. 1999;18:393-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mupirocin resistance: Clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother. 2015;70:2681-92.
- [CrossRef] [PubMed] [Google Scholar]
- Mupirocin resistance among methicillin-resistant Staphylococcus aureus-colonized patients at admission to a tertiary care medical center. J Clin Microbiol. 2009;47:2279-80.
- [CrossRef] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. PubChem Database Ozenoxacin, CID=9863827. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Ozenoxacin [Last accessed on 2020 Feb 19]
- [Google Scholar]
- Ozenoxacin: A novel drug discovery for the treatment of impetigo. Curr Drug Discov Technol. 2019;16:259-64.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro activity of ozenoxacin against quinolone-susceptible and quinolone-resistant Gram-positive bacteria. Antimicrob Agents Chemother. 2013;57:6389-92.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanism of action of and resistance to quinolones. Microb Biotechnol. 2009;2:40-61.
- [CrossRef] [PubMed] [Google Scholar]
- Activities of gyrase and topoisomerase IV on positively supercoiled DNA. Nucleic Acids Res. 2017;45:9611-24.
- [CrossRef] [PubMed] [Google Scholar]
- DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377-92.
- [CrossRef] [PubMed] [Google Scholar]
- DNA topoisomerase: The key enzyme that regulates DNA super structure. Nagoya J Med Sci. 1998;61:11-26.
- [Google Scholar]
- Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob Agents Chemother. 2001;45:3544-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ozenoxacin: A review of preclinical and clinical efficacy. Expert Rev Anti Infect Ther. 2019;17:159-68.
- [CrossRef] [PubMed] [Google Scholar]
- Ozenoxacin: A novel topical quinolone for impetigo. Ann Pharmacother. 2018;52:1233-7.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro and in vivo antibacterial activity of T-3912, a novel non-fluorinated topical quinolone. J Antimicrob Chemother. 2002;49:455-65.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative in vitro antibacterial activity of ozenoxacin against Gram-positive clinical isolates. Future Microbiol. 2018;13:3-19.
- [CrossRef] [PubMed] [Google Scholar]
- Ozenoxacin 1% cream in the treatment of impetigo: A multicenter, randomized, placebo-and retapamulin-controlled clinical trial. Future Microbiol. 2014;9:1013-23.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized vehicle-controlled trial to assess the efficacy, safety, and tolerability of ozenoxacin 1% cream in 465 patients 2 years and older with impetigo. J Cutan Med. 2017;1:s104.
- [CrossRef] [Google Scholar]
- Therapeutic efficacy of ozenoxacin in animal models of dermal infection with Staphylococcus aureus. Future Microbiol. 2018;13:21-30.
- [CrossRef] [PubMed] [Google Scholar]
- Accumulation of ozenoxacin and other quinolones in Gram-positive bacteria. Enferm Infecc Microbiol Clin. 2018;36:146.
- [Google Scholar]
- Ozanex (Ozenoxacin): 1% w/w Topical Antibiotic Cream [Product Monograph] Barcelona (ES): Ferrer Internacional, S.A..
- [Google Scholar]
- Impact of efflux in the development of multidrug resistance phenotypes in Staphylococcus aureus. BMC Microbiol. 2015;15:232.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic bioavailability, safety and tolerability of topical ozenoxacin in healthy adult volunteers. Future Microbiol. 2014;9:S11-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of ozenoxacin cream for treatment of adult and pediatric patients with impetigo: A randomized clinical trial. JAMA Dermatol. 2018;154:806-13.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Review Report: Ozenoxacin 1% Cream (Ozanex) Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018.
- [Google Scholar]
- Ozenoxacin, a new effective and safe topical treatment for impetigo in children and adolescents. Dermatology. 2020;236:199-207.
- [CrossRef] [PubMed] [Google Scholar]
- Cumulative irritation, sensitizing potential, phototoxicity and photoallergy of ozenoxacin in healthy adult volunteers. Future Microbiol. 2014;9:S23-31.
- [CrossRef] [PubMed] [Google Scholar]
- Studies on articular and general toxicity of orally administered ozenoxacin in juvenile rats and dogs. Future Microbiol. 2018;13:31-40.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic bioavailability and safety of twice-daily topical ozenoxacin 1% cream in adults and children with impetigo. Future Microbiol. 2014;9:S33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Ferrer Internacional, S. A Pharmacoeconomic Review Report: Ozenoxacin 1% Cream (Ozanex) Indication: The Topical Treatment of Impetigo in Patients Aged Two Months and Older. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; October, 2018 Appendix 1, Cost Comparison. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539348/ [Last accessed on 2020 Jun 10]
- [Google Scholar]





